Catalog No.
KDV02801
Description
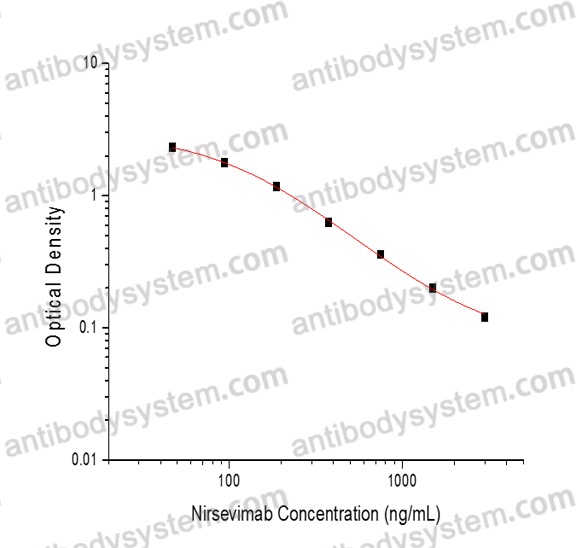
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human respiratory syncytial virus A (HRSV) Fusion glycoprotein F0 has been pre-coated onto a microplate. Standards or samples are premixed with HRP-labeled antibody and then pipetted into the wells. Nirsevimab in the sample competitively binds to the pre-coated protein with HRP-labeled Nirsevimab. After washing away any unbound substances, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Nirsevimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Nirsevimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
46.88 - 3,000 ng/mL
Sensitivity
13.72 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1603.5
|
294.5
|
80.3
|
876.0
|
248.9
|
80.7
|
|
Standard deviation
|
305.2
|
11.6
|
4.6
|
167.3
|
22.1
|
10.1
|
|
CV (%)
|
19.0
|
3.9
|
5.7
|
19.1
|
8.9
|
12.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MEDI-8897, MEDI8897, CAS: 1989556-22-0
Effectiveness and impact of nirsevimab in Chile during the first season of a national immunisation strategy against RSV (NIRSE-CL): a retrospective observational study., PMID:40513593
Respiratory Syncytial Virus: A Narrative Review of Updates and Recent Advances in Epidemiology, Pathogenesis, Diagnosis, Management and Prevention., PMID:40507642
Nirsevimab: a change of paradigm in the prophylaxis of Respiratory Syncytial Virus disease among infants., PMID:40503651
Effectiveness of nirsevimab against RSV-related outcomes: findings of the 2024-2025 campaign in Catalonia align with previous analysis., PMID:40499942
Scenario Projections of Respiratory Syncytial Virus Hospitalizations Averted Due to New Immunizations., PMID:40498487
Evaluation of pregnant women's knowledge about RSV and immunization attitudes before infant immunization with monoclonal antibodies in Turkey., PMID:40489965
Demographic Characteristics Associated With Uptake of Neonatal Respiratory Syncytial Virus Prophylaxis., PMID:40489377
Strategies to improve interprofessional communication regarding maternal and infant RSV immunization., PMID:40483884
Nirsevimab Provides Real-World RSV Protection in Infants., PMID:40478586
Intention to Use RSVpreF Vaccine or Nirsevimab to Prevent Infant RSV Among Pregnant Individuals., PMID:40472256
Respiratory syncytial virus prophylaxis for children in Africa: Challenges, opportunities and public health strategies., PMID:40469396
Effectiveness of immunization strategies for preventing severe acute respiratory infection during the 2023/2024 season in a Spanish health department., PMID:40467410
Turkish pediatricians' knowledge, attitudes, and awareness of respiratory syncytial virus (RSV) infection and immunization strategies: a cross-sectional study., PMID:40466672
The potential public health impact of new immunization strategies for the prevention of RSV in children in Panama., PMID:40458968
Administration of Nirsevimab for RSV Prophylaxis in Infants: A Comprehensive Review., PMID:40432081
Impact of Nirsevimab immunoprophylaxis on professional exhaustion during two epidemics of respiratory syncytial virus., PMID:40414172
Vectored long-term co-delivery of antibodies for SARS-CoV-2, RSV and Influenza prophylaxis., PMID:40413832
Respiratory syncytial virus preventives for children in Australia: current landscape and future directions., PMID:40413643
"Maternal Perspectives on Decision-Making for Newborn RSV Prophylaxis with Nirsevimab"., PMID:40413636
Health-care burden related to respiratory syncytial virus in a resource-constrained setting: a prospective observational study., PMID:40412396
Estimated impact of nirsevimab prophylaxis on the economic burden of respiratory syncytial virus disease in Northern Italy., PMID:40400006
Cost-effectiveness analysis of nirsevimab for prevention of respiratory syncytial virus disease among infants in Shanghai, China: A modeling study., PMID:40391452
180-day efficacy of nirsevimab against hospitalisation for respiratory syncytial virus lower respiratory tract infections in infants (HARMONIE): a randomised, controlled, phase 3b trial., PMID:40379431
Update on Routine Immunizations for Children and Adolescents., PMID:40378322
Universal administration of nirsevimab in infants: an analysis of hospitalisations and paediatric intensive care unit admissions for RSV-associated lower respiratory tract infections., PMID:40377714
Direct Out-of-Pocket Costs of Nirsevimab vs. Palivizumab during the First Respiratory Syncytial Virus Season: A Comparative Analysis., PMID:40343422
RSV: an update on prevention and management., PMID:40343137
Estimating the impact of Western Australia's first respiratory syncytial virus immunisation program for all infants: A mathematical modelling study., PMID:40339485
Interim Evaluation of Respiratory Syncytial Virus Hospitalization Rates Among Infants and Young Children After Introduction of Respiratory Syncytial Virus Prevention Products - United States, October 2024-February 2025., PMID:40338822
Knowledge, attitudes, and practices of Croatian pediatricians and pediatrics residents about RSV infection., PMID:40328920
Infant Respiratory Syncytial Virus Immunization Coverage in the Vaccine Safety Datalink: 2023-2024., PMID:40324788
Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: a systematic review and meta-analysis., PMID:40319903
Cost-Effectiveness Analysis of Nirsevimab for the Prevention of Respiratory Syncytial Virus among Italian Infants., PMID:40317387
Respiratory syncytial virus-related lower respiratory tract infection hospitalizations in infants receiving nirsevimab in Galicia (Spain): the NIRSE-GAL study., PMID:40314706
Effectiveness of nirsevimab immunization against RSV infection in preterm infants: a systematic review and meta-analysis., PMID:40313952
Editorial: Surveillance of Seasonal Respiratory Syncytial Virus (RSV) Infection in Children and Vulnerable Adults Drives Vaccine Development and New Immunization Programs., PMID:40308086
Nirsevimab and Maternal Respiratory Syncytial Virus Vaccine Recommendations for the Pediatric Population., PMID:40305635
Impact of Nirsevimab on RSV and Non-RSV Severe Respiratory Infections in Hospitalized Infants., PMID:40302169
Nirsevimab immunisation of infants and respiratory syncytial virus (RSV)-associated hospitalisations, Western Australia, 2024: a population-based analysis., PMID:40293046
Nirsevimab prophylaxis on pediatric intensive care hospitalization for severe acute bronchiolitis: a clinical and economic analysis., PMID:40281363
Human iPS cell-derived respiratory organoids as a model for respiratory syncytial virus infection., PMID:40262853
Past, present and future of respiratory syncytial infection prevention in infants and young children., PMID:40243138
Global Pediatric Pulmonology Alliance recommendations to protect all infants against respiratory syncytial virus with prophylactic monoclonal antibodies., PMID:40241887
Summary of the National Advisory Committee on Immunization (NACI) Statement on the Prevention of Respiratory Syncytial Virus (RSV) in Infants., PMID:40241712
Navigating parental hesitancy in public health: the case for RSV immunization in newborns., PMID:40216995
Impact of routine prophylaxis with monoclonal antibodies and maternal immunisation to prevent respiratory syncytial virus hospitalisations, Lombardy region, Italy, 2024/25 season., PMID:40211969
Projecting maximum potential demand for nirsevimab to protect eligible US infants and young children against respiratory syncytial virus in the 2024/2025 season., PMID:40209626
[New developments in the prevention and management of RSV in 2025]., PMID:40208117
Sociodemographic Characteristics of Infants Receiving Nirsevimab., PMID:40202762
Health economic evaluation of implementing a universal immunization program with nirsevimab compared to standard of care for the prevention of respiratory syncytial virus disease in Canadian infants., PMID:40186452