Catalog No.
KDJ70102
Description
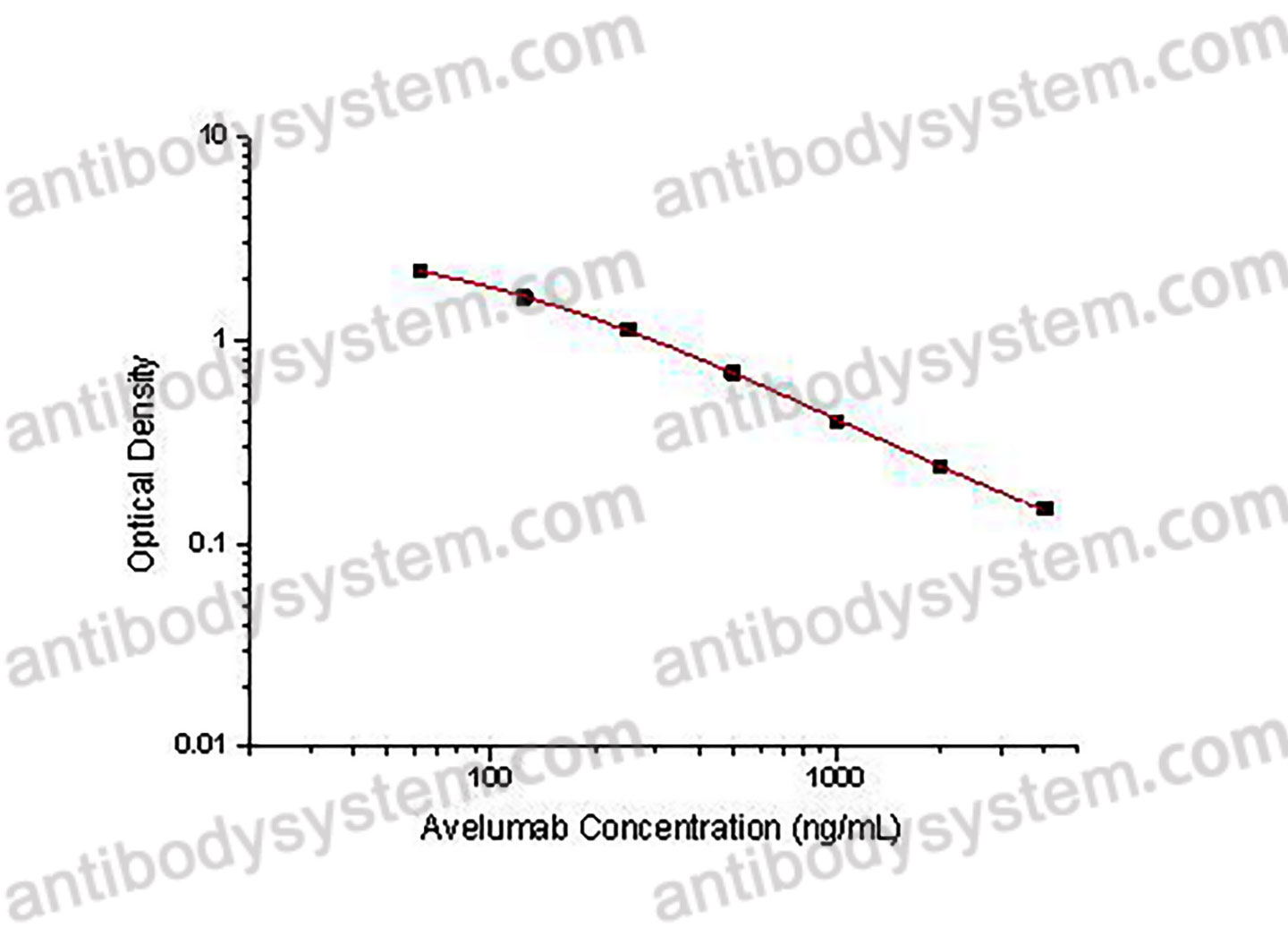
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD274 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Avelumab in the sample competitively binds to the pre-coated protein with biotin-labeled Avelumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Avelumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Avelumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
18.50 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1724.8
|
427.8
|
100.8
|
2011.1
|
499.0
|
112.5
|
|
Standard deviation
|
129.8
|
26.1
|
5.1
|
162.1
|
30.0
|
11.8
|
|
CV (%)
|
7.5
|
6.1
|
5.1
|
8.1
|
6.0
|
10.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MSB-0010718C, MSB0010682, , CAS: 1537032-82-8
Background
Avelumab (Bavencio®) is a fully human anti-PD-L1 IgG1 lambda monoclonal antibody that has a molecular weight of approximately 147 kDa. Avelumab binds PD-L1 and blocks the interaction between PD-L1 and its receptors PD-1 and B7-1. By inhibiting PD-L1 interactions, avelumab is thought to enable the activation of T-cells and the adaptive immune system. By retaining anative Fc-region, avelumab is thought to engage the innate immune system and may induce antibody-dependent cell-mediated cytotoxicity. Importantly, avelumab has not shown antibody-dependent cell mediated cytotoxicity against immune cell subsets in humans.
Avelumab maintenance in advanced urothelial carcinoma: real-world data from Northern Spain (AVEBLADDER study)., PMID:40514610
Avelumab plus axitinib in patients with advanced gastrointestinal stromal tumor., PMID:40499273
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
Clinical features, treatment, and outcomes of anti-PD-L1 induced psoriasis., PMID:40474015
Avelumab First-line Maintenance for Advanced Urothelial Carcinoma: Long-term Outcomes from the JAVELIN Bladder 100 Trial in Patients with Nonvisceral or Lymph Node-only Disease., PMID:40467365
Avelumab plus sacituzumab govitecan versus avelumab monotherapy as first-line maintenance treatment in patients with advanced urothelial carcinoma: JAVELIN Bladder Medley interim analysis., PMID:40456670
Avelumab and taxol chemotherapy in platinum-refractory or ineligible metastatic urothelial carcinoma (AVETAX trial)., PMID:40451703
Phase II (Alliance A091802) Randomized Trial of Avelumab Plus Cetuximab Versus Avelumab Alone in Advanced Cutaneous Squamous Cell Carcinoma., PMID:40448574
A case of irAE myositis with positive anti-striational antibodies after anti-PD-L1 antibody administration: a case report., PMID:40445182
Avelumab-Induced Ocular Myasthenia Gravis: A Case Report., PMID:40416255
Avelumab-based neoadjuvant therapy in patients with muscle-invasive bladder cancer (AURA Oncodistinct-004): a phase 2 multicenter clinical trial., PMID:40413024
Impact of rheumatoid factors on the function of therapeutic monoclonals specific for PD-1/PD-L1., PMID:40411581
Interplay between pharmacokinetics and immunogenicity of therapeutic proteins: stepwise development of a bidirectional joint pharmacokinetics-anti-drug antibodies model., PMID:40399699
Comment on "Phase I/II Trial of Perioperative Avelumab in Combination With Chemoradiation in the Treatment of Stage II/III Resectable Esophageal and Gastroesophageal Junction Cancer"., PMID:40396495
Phase 2, two-stage study of avelumab and axitinib in patients with mismatch repair proficient recurrent or persistent endometrial cancer., PMID:40393272
Phase II study of perioperative Avelumab plus chemotherapy for patients with resectable gastric cancer or gastroesophageal junction cancer - the MONEO Study., PMID:40388549
A Cost-Effectiveness Analysis for Avelumab as a First-Line Maintenance Treatment of Advanced Urothelial Carcinoma in the Netherlands., PMID:40381164
Response and Survival With Immune Checkpoint Inhibitor in Patients With Advanced Urothelial Carcinoma and Histology Subtypes., PMID:40378559
Evaluation of Optimal Sequential Treatment Patterns and Clinical Outcomes in Patients With Advanced Urothelial Carcinoma Treated With First-Line Platinum-Based Chemotherapy: A Multicenter Collaborative Study., PMID:40375459
Additional immunotherapy to standard of care for unresectable locally advanced head and neck squamous cell carcinoma: a meta-analysis., PMID:40373754
Autoencoder techniques for survival analysis on renal cell carcinoma., PMID:40373089
Editorial Comment to "Serum Lactate Dehydrogenase Level Prior to First-Line Chemotherapy for Metastatic Urothelial Carcinoma is a Prognostic Factor for Avelumab Maintenance Therapy: A Multicenter Retrospective Study"., PMID:40371448
Association of Sinonasal Symptoms and Disease With Immune Checkpoint Inhibitor Therapy., PMID:40370338
Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions., PMID:40361519
Haematological toxicities with immune checkpoint inhibitors in digestive system tumors: a systematic review and network meta-analysis of randomized controlled trials., PMID:40360867
Co-Occurrence of Hypophysitis and Thyroiditis Induced by PD-L1 Inhibitor Avelumab: Clinical Insights., PMID:40356788
Accelerated atherosclerosis associated with immune checkpoint inhibitors: a systematic review and meta-analysis of pre-clinical studies., PMID:40354680
Complete Response after Avelumab Maintenance Therapy: Successful Management of Metastatic Urothelial Carcinoma., PMID:40352709
A Case of Immune-Related Adverse Events, Including Myasthenia Gravis, Myositis, and Myocarditis, during Avelumab and Axitinib Combination Therapy., PMID:40351546
Xevinapant plus avelumab in advanced solid tumours, with a dose expansion in advanced non-small-cell lung cancer: exploratory biomarker, safety and efficacy analyses from an open-label, nonrandomised phase Ib study., PMID:40351326
Serum Lactate Dehydrogenase Level Prior to First-Line Chemotherapy for Metastatic Urothelial Carcinoma Is a Prognostic Factor for Avelumab Maintenance Therapy: A Multicenter Retrospective Study., PMID:40329662
Efficacy of First-Line Treatments for Advanced Renal Cell Carcinoma: A Bayesian Network Meta-analysis of Objective Response, Progression-Free Survival, and Overall Survival., PMID:40329046
Liver transplantation for hepatocellular carcinoma following immunotherapy., PMID:40326429
Avelumab First-line Maintenance for Advanced Urothelial Carcinoma: Long-term Analyses of Patient-reported Outcomes and Quality-adjusted Time Without Symptoms or Toxicity from the JAVELIN Bladder 100 Trial., PMID:40318950
Avelumab induces greater Fc-Fc receptor-dependent natural killer cell activation and dendritic cell crosstalk compared to durvalumab., PMID:40311014
A Phase II Randomized Study of Paclitaxel alone or combined with Pelareorep with or without Avelumab in Metastatic Hormone Receptor Positive Breast Cancer: the BRACELET-01/PrE0113 study., PMID:40300087
A Real-World Pharmacovigilance Study of FDA Adverse Event Reporting System Events for Avelumab., PMID:40291233
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System., PMID:40290514
Short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced microsatellite stable rectal adenocarcinoma: The Averectal study., PMID:40286473
Real-World Clinical Outcomes with First-Line Systemic Treatment and Avelumab Maintenance in US Patients with Locally Advanced or Metastatic Urothelial Carcinoma: The SPEAR Bladder-II Study., PMID:40277744
Association of peripheral monocytic myeloid-derived suppressor cells with molecular subtypes in single-center endometrial cancer patients receiving carboplatin + paclitaxel/avelumab (MITO-END3 trial)., PMID:40244420
Grade 3 and Grade 4 Cutaneous toxicities in patients across multiple solid tumour types receiving checkpoint inhibitor therapy: An observational study. The experience of a single large specialist institution., PMID:40241399
Phase 2 trial of perioperative chemo-immunotherapy for gastro-esophageal adenocarcinoma: The role of M2 macrophage landscape in predicting response., PMID:40239627
Assessment of Immunoscore, MRI Tumor Regression Grade, and Neoadjuvant Rectal Score in Predicting Pathologic Response in Locally Advanced Rectal Cancer in the Averectal Study., PMID:40218263
Immune check point inhibitors for ocular adnexal and periocular tumors., PMID:40213294
Regorafenib plus avelumab in advanced gastroenteropancreatic neuroendocrine neoplasms: a phase 2 trial and correlative analysis., PMID:40204996
Real-world avelumab first-line maintenance in advanced urothelial carcinoma: systematic review and meta-analysis., PMID:40184226
Prediction, prevention, and precision treatment of immune checkpoint inhibitor neurological toxicity using autoantibodies, cytokines, and microbiota., PMID:40181971
Cisplatin-induced therapy-related myelodysplastic syndrome during avelumab maintenance therapy for metastatic urothelial carcinoma., PMID:40160873
A phase I/II trial of avelumab combinations with ivuxolimab, utomilumab, and radiation therapy in patients with advanced gastrointestinal malignancies., PMID:40139261