Catalog No.
KDJ70101
Description
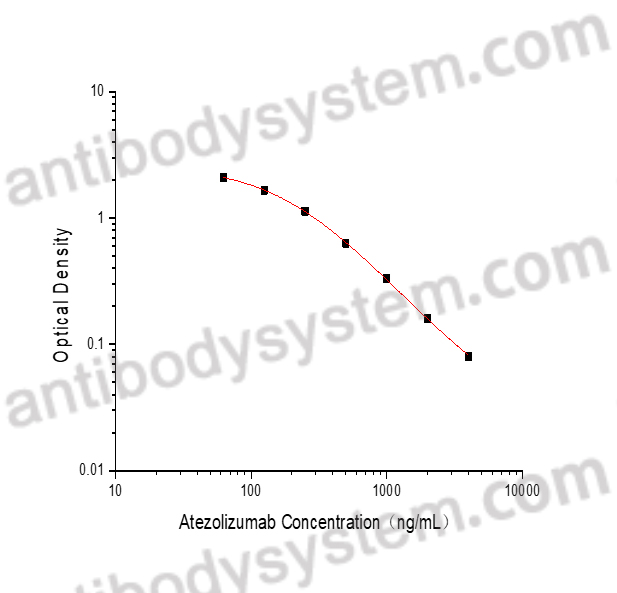
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. A recombinant human PD-L1 antigen has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Atezolizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Atezolizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Atezolizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Atezolizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
31.82 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1909.9
|
531.6
|
127.0
|
2039.9
|
580.1
|
131.2
|
|
Standard deviation
|
73.1
|
14.0
|
4.1
|
154.6
|
26.1
|
6.0
|
|
CV (%)
|
3.8
|
2.6
|
3.2
|
7.6
|
4.5
|
4.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MPDL3280A, RG7446, CAS: 1380723-44-3
Background
Atezolizumab (trade name Tecentriq) is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1). In 2015, it was in clinical trials as an immunotherapy for several types of solid tumors. It was under investigation by Genetech/Roche. In April 2016, Roche announced that atezolizumab had been granted fast track status for lung cancer by the FDA. In May 2018, Tecentriq was in combination with Avastin and standard chemotherapy for some patients with lunch cancer was granted priority review.
Advances in targeted therapy for triple-negative breast cancer: a review of key antigens and recent advances., PMID:40515614
Real-World Experiences Using Atezolizumab + Bevacizumab for the Treatment of Unresectable Hepatocellular Carcinoma: A Multicenter Study., PMID:40507295
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Gender and age disparities in cardiac immune-related adverse events associated with immune checkpoint inhibitors: a pharmacovigilance analysis of the FAERS database., PMID:40506075
Predicting atezolizumab response in metastatic urothelial carcinoma patients using machine learning on integrated tumour gene expression and clinical data., PMID:40494842
Atezolizumab plus bevacizumab versus Lenvatinib for patients with Barcelona clinic liver cancer stage B (BCLC-B) hepatocellular carcinoma (HCC): A real-world population., PMID:40494131
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
LMNB2-mediated high PD-L1 transcription triggers the immune escape of hepatocellular carcinoma., PMID:40483310
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
Clinical features, treatment, and outcomes of anti-PD-L1 induced psoriasis., PMID:40474015
The current and emerging immunotherapy paradigm in small-cell lung cancer., PMID:40473974
Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer (IMforte): a randomised, multicentre, open-label, phase 3 trial., PMID:40473449
Delayed central nervous system progression with atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer (LU23-15)., PMID:40473339
Immune checkpoint inhibitor-induced myocarditis and multiple adverse events with pre-existing rheumatoid arthritis: a case report and literature review., PMID:40471689
Complete Response of Hepatocellular Carcinoma With Inferior Vena Cava Tumor Thrombus and Right Atrium Involvement to Combined Radiotherapy and Immunotherapy: A Case Report., PMID:40470485
Feasibility and outcome of genomics-guided treatment selection in advanced cancer - the MEGALiT explorative clinical trial., PMID:40468525
Peri-operative atezolizumab in early-stage triple-negative breast cancer: final results and ctDNA analyses from the randomized phase 3 IMpassion031 trial., PMID:40467898
Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: the ATRACTIB phase 2 trial., PMID:40467896
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Comparison Analysis of Lenvatinib Plus Transcatheter Arterial Chemoembolization Versus Atezolizumab Plus Bevacizumab as First-Line Therapy for Intermediate-Stage Hepatocellular Carcinoma Beyond the Up-to-Seven Criteria., PMID:40448543
Treatment of small cell lung cancer; advances and future prospects., PMID:40446464
Five-Year Survival Outcomes With Atezolizumab After Chemotherapy in Resected Stage IB-IIIA Non-Small Cell Lung Cancer (IMpower010): An Open-Label, Randomized, Phase III Trial., PMID:40446184
Precision targeting of β-catenin induces tumor reprogramming and immunity in hepatocellular cancers., PMID:40442146
Efficacy and Safety of Lenvatinib versus Atezolizumab Plus Bevacizumab in the Treatment of Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis., PMID:40439364
Efficacy and safety of BCG and immune checkpoint inhibitors in non-muscle invasive bladder cancer: A meta-analysis with exploratory chemotherapy comparisons., PMID:40438876
Matching-Adjusted Indirect Comparison of Arterial FOLFOX and Atezolizumab-Bevacizumab in Unresectable Hepatocellular Carcinoma., PMID:40438087
Circulating tumor DNA monitoring and blood tumor mutational burden in patients with metastatic solid tumors treated with atezolizumab., PMID:40434907
Intrahepatic Lymphoid Follicles Comprising T and B Cells Mimic Hepatocellular Carcinoma in a Hepatitis B Patient., PMID:40429964
Neuroendocrine Neoplasms of the Lungs, Thyroid, and Thymus., PMID:40426858
Which sarcoma requires PD1/PDL1 inhibitors, and what should be the best scheme? Present status and next steps., PMID:40421971
Number of Tumors Stratifies the Therapeutic Response to Atezolizumab Plus Bevacizumab Therapy in Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: A Multicenter Analysis., PMID:40418927
Immunotherapy in Triple-Negative Breast Cancer., PMID:40418298
The bispecific antibody targeting VISTA and PD-L1 shows enhanced tumor inhibitory activity in pancreatic, endometrial and breast cancers compared to mono- and combination immune checkpoint blockade., PMID:40416959
Looking Toward the Future: Emerging Therapies for Hepatocellular Carcinoma., PMID:40416920
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
Adjuvant Immunotherapy in Microsatellite Instability-High Colon Cancer: A Literature Review on Efficacy, Challenges, and Future Directions., PMID:40415356
Subset of Child-Pugh Score 7 Shows Comparable Survival Outcomes to Child-Pugh Score 6 in Hepatocellular Carcinoma Patients Treated with Atezolizumab and Bevacizumab., PMID:40407727
Urine Tumor DNA to Stratify the Risk of Recurrence in Patients Treated with Atezolizumab for Bacillus Calmette-Guérin-unresponsive Non-muscle-invasive Bladder Cancer., PMID:40404526
Development and validation of a serum proteomic test for predicting patient outcomes in advanced non-small cell lung cancer treated with atezolizumab or docetaxel., PMID:40404206
Atezolizumab Plus Bevacizumab Combined with or without Transarterial Chemoembolization in the Treatment of Advanced Hepatocellular Carcinoma: A Single-Center Retrospective Study., PMID:40395491
Photoimmuno-Lure Nanoplatform for Enhancing T Cell Expansion in Glioblastoma via Synergistic Treatment of Photodynamic Therapy and Immune Checkpoint Inhibition., PMID:40395101
A plain language summary of atezolizumab compared with single-agent chemotherapy in participants with non-small cell lung cancer who should not receive platinum-based chemotherapy (the IPSOS study)., PMID:40391393
Biomarker, efficacy and safety analysis of transcatheter arterial chemoembolization combined with atezolizumab and bevacizumab for unresectable hepatocellular carcinoma., PMID:40387956
Separation of simultaneously acquired [89Zr]atezolizumab and [18F]FDG PET scans., PMID:40387909
Immune-mediated adverse events following atezolizumab and bevacizumab in a multinational Latin American cohort of unresectable hepatocellular carcinoma., PMID:40387836
Effect of the number of induction chemotherapy cycles on the efficacy of first-line atezolizumab combined with chemotherapy in extensive-stage small cell lung cancer., PMID:40386714
Cost-effectiveness of atezolizumab versus chemotherapy in patients with non-small-cell lung cancer ineligible for platinum-based doublet chemotherapy., PMID:40385627
Systemic Therapy Combined with Locoregional Therapy in Intermediate-stage Hepatocellular Carcinoma., PMID:40384918
Atezolizumab plus bevacizumab and chemotherapy in metastatic nonsquamous NSCLC: the randomized double-blind phase 3 IMpower151 trial., PMID:40379995
Atezolizumab and Stereotactic Body Radiation in Metastatic, Recurrent, or Persistent Cervical Cancer: Results From a Phase 2 Multi-Institutional Study., PMID:40379142