Catalog No.
KDJ63101
Description
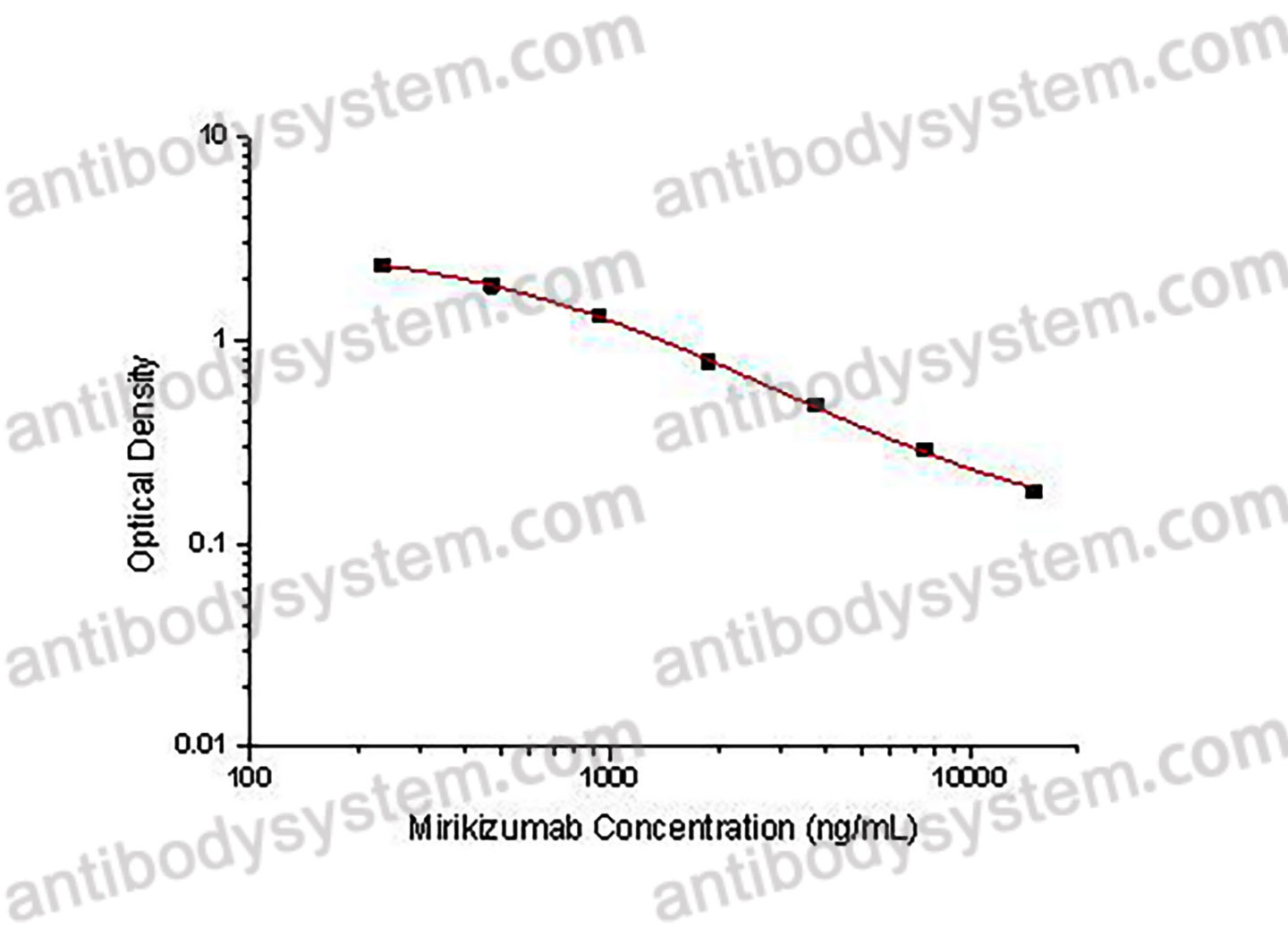
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL23A has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Mirikizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Mirikizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Mirikizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Mirikizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
78.23 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
8257.1
|
1990.4
|
471.8
|
7736.7
|
1743.9
|
431.3
|
|
Standard deviation
|
596.3
|
148.3
|
35.6
|
907.0
|
207.7
|
52.2
|
|
CV (%)
|
7.2
|
7.5
|
7.5
|
11.7
|
11.9
|
12.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
LY-3074828, CAS: 1884201-71-1
Background
Mirikizumab (Eli Lilly and Company), Risankizumab (Boehringer Ingelheim and AbbVie), Tildrakizumab (Sun Pharma), Brazikumab (AstraZeneca), and Guselkumab (Janssen Biotech Inc.) are other drugs which target IL-23 and received the FDA approval for the treatment of psoriasis.
Impact of Mirikizumab Treatment on Fatigue in Patients with Moderately to Severely Active Crohn's Disease: Results from the Phase 3 VIVID-1 Study., PMID:40491246
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
IL-17A inhibitor-induced ulcerative colitis treated with an anti-IL-23 antibody., PMID:40455166
First Documented Case of Successful Dual Therapy With Upadacitinib and Mirikizumab for Multi-Refractory Ulcerative Proctitis., PMID:40411442
With guselkumab, does the dual mechanisms to inhibit IL-23, help in ulcerative colitis?, PMID:40394835
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Editorial: Real-World Effectiveness of Mirikizumab in Ulcerative Colitis-A Valuable but Preliminary Glimpse. Authors' Reply., PMID:40321062
Editorial: Real-World Effectiveness of Mirikizumab in Ulcerative Colitis-A Valuable but Preliminary Glimpse., PMID:40321049
Efficacy and safety of Mirikizumab for ulcerative colitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40301737
Efficacy and safety of mirikizumab in the treatment of inflammatory bowel disease: A meta-analysis., PMID:40295305
New Interleukin-23 Antagonists' Use in Crohn's Disease., PMID:40283885
Efficacy and Safety of Mirikizumab in the Treatment of Moderately to Severely Active Ulcerative Colitis Regardless of Baseline Modified Mayo Score: Results From the Phase 3 LUCENT Trials., PMID:40260308
Mirikizumab for Ulcerative Colitis: A Game-Changer or Just Another Incremental Advance?, PMID:40237908
Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients With Ulcerative Colitis: A Multicentre Retrospective Observational Study., PMID:40205711
Efficacy of Mirikizumab in Patients with Prior Ustekinumab Exposure: A Case Series., PMID:40198002
Pharmacokinetic Comparability and Safety Between Original and Citrate-Free Mirikizumab Formulations for Subcutaneous Injections: Results from Three Clinical Trials., PMID:40117091
Paradigm Shift in Inflammatory Bowel Disease Management: Precision Medicine, Artificial Intelligence, and Emerging Therapies., PMID:40095460
IL23p19 therapies for moderately-to-severely active ulcerative colitis., PMID:40082083
Mirikizumab Improves Quality of Life and Work Productivity in Patients with Moderately to Severely Active Crohn's Disease: Results from the Phase 3 VIVID-1 Study., PMID:40079470
Long-Term Safety of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis: An Integrated 2-Year Safety Analysis., PMID:40071779
Combining mechanistic modeling with machine learning as a strategy to predict inflammatory bowel disease clinical scores., PMID:40070575
Guselkumab (Tremfya) for ulcerative colitis., PMID:40053377
Mirikizumab in the Treatment of Ulcerative Colitis: Initial Real-World Data in a Population from a Large Tertiary Center., PMID:40048134
Drug Development in Inflammatory Bowel Diseases: What Is Next?, PMID:40006003
Epidemiology, pathogenesis, diagnosis, and treatment of inflammatory bowel disease: Insights from the past two years., PMID:39994836
The VIVID-1 study: A novel methodological approach provides further evidence of efficacy of selective IL-23 inhibition in Crohn's disease., PMID:39954670
Efficacy and safety of mirikizumab in psoriasis: a systematic review and meta-analysis of randomized controlled trials., PMID:39954188
Role of Mirikizumab in the Treatment of Inflammatory Bowel Disease-From Bench to Bedside., PMID:39941671
Mirikizumab for Crohn's Disease: Small Step Forward or Giant Leap Ahead?, PMID:39914777
Long-Term Endoscopic and Histological Outcomes of Mirikizumab in Patients With Moderately to Severely Active Ulcerative Colitis With Up to 3 Years of Treatment., PMID:39896691
Exit Interviews Exploring Patients' Experience of Change in Crohn's Disease Symptoms During the Mirikizumab Phase 3 Clinical Trial In Adult Patients With Moderately-to-Severely Crohn's Disease., PMID:39834356
Mirikizumab pharmacokinetics and exposure-response in pediatric patients with moderate-to-severe ulcerative colitis., PMID:39693227
The Urgency Numeric Rating Scale: Psychometric Evaluation in Adults with Crohn's Disease., PMID:39692838
Targeting mucosal healing in Crohn's disease: efficacy of novel pathways and therapeutic targets., PMID:39611536
Efficacy and safety of mirikizumab in patients with moderately-to-severely active Crohn's disease: a phase 3, multicentre, randomised, double-blind, placebo-controlled and active-controlled, treat-through study., PMID:39581202
AGA Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis., PMID:39572132
Mirikizumab for Chronic Plaque Psoriasis: A Systematic Review and Meta-Analysis., PMID:39570737
Risankizumab (Skyrizi) for ulcerative colitis., PMID:39509158
Patient and Healthcare Professional Satisfaction, Acceptability, and Preference Experiences With Mirikizumab Administration for Ulcerative Colitis: An International Survey., PMID:39473630
Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study., PMID:39448057
Comparative Efficacy of Advanced Therapies for Management of Moderate-to-Severe Ulcerative Colitis: 2024 American Gastroenterological Association Evidence Synthesis., PMID:39425738
Efficacy and Safety of Advanced Therapies in Moderately-to-Severely Active Ulcerative Colitis: a Systematic Review and Network Meta-analysis., PMID:39404996
Network Meta-Analysis: Histologic and Histo-Endoscopic Improvement and Remission With Advanced Therapy in Ulcerative Colitis., PMID:39367678
An evaluation of mirikizumab for the treatment of ulcerative colitis., PMID:39360778
Mirikizumab for the treatment of chronic antibiotic-refractory pouchitis., PMID:39321965
Efficacy and safety of mirikizumab compared with currently approved biologic drugs for the treatment of ulcerative colitis: A systematic review and network meta-analysis., PMID:39320112
Recent clinical evidence on nutrition, novel pharmacotherapy, and vaccination in inflammatory bowel diseases., PMID:39308999
Safety of Rotavirus Vaccination in Infants That Were Exposed to Biologics In Utero: A Systematic Review., PMID:39303214