Catalog No.
KDJ48701
Description
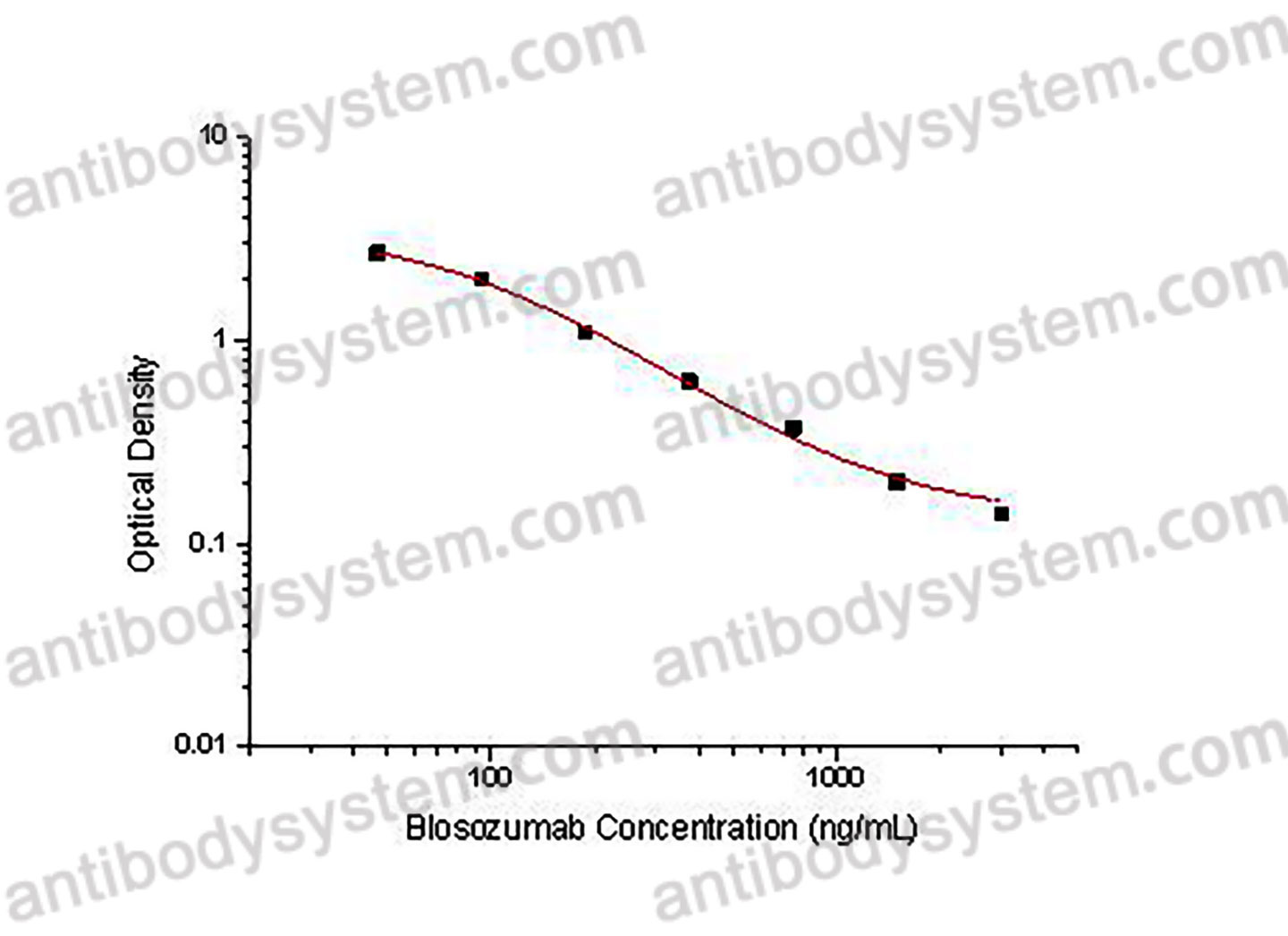
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human SOST has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Blosozumab in the sample competitively binds to the pre-coated protein with biotin-labeled Blosozumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Blosozumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Blosozumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
46.88 - 3,000 ng/mL
Sensitivity
38.87 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
1751.7 |
473.5 |
111.3 |
2348.0 |
496.8 |
110.4 |
|
Standard deviation |
129.0 |
18.8 |
6.1 |
347.4 |
30.8 |
8.0 |
|
CV (%) |
7.4 |
4.0 |
5.5 |
14.8 |
6.2 |
7.3 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
LY2541546, CAS: 1132758-87-2
Background
Blosozumab is an IgG4-kappa type mouse monoclonal antibody optimized by humanization engineering with a molecular weight of 144.63 kDa. The monoclonal antibody was developed by Eli Lilly for the treatment of osteoporosis. Blosozumab can target and bind sclerostin (SOST) to play a biological role. In 2014, blosozumab was conducted a different doses of clinical trial about blosozumab was conducted to evaluate including drug safety, tolerance, pharmacokinetics and pharmacodynamics in postmenopausal women. The results showed that blosozumab had well tolerance and anabolic effect on bone, which could be used as a potential treatment for osteoporosis. In 2015, a randomized double-blind phase Ⅱ trial of blosozumab in postmenopausal women with low bone mineral density showed that blosozumab treatment resulted in a significant dose-dependent increase in bone mineral density, which further supports the conclusion that blosozumab is a potential anabolic therapy for osteoporosis. In September of the same year, women who had completed treatment were followed up to evaluate the effect of discontinuation of blosozumab. It was found that there were no adverse events related to the treatment one year after the cessation of treatment, and the anti-drug antibodies in the patients generally decreased.