Catalog No.
KDJ36101
Description
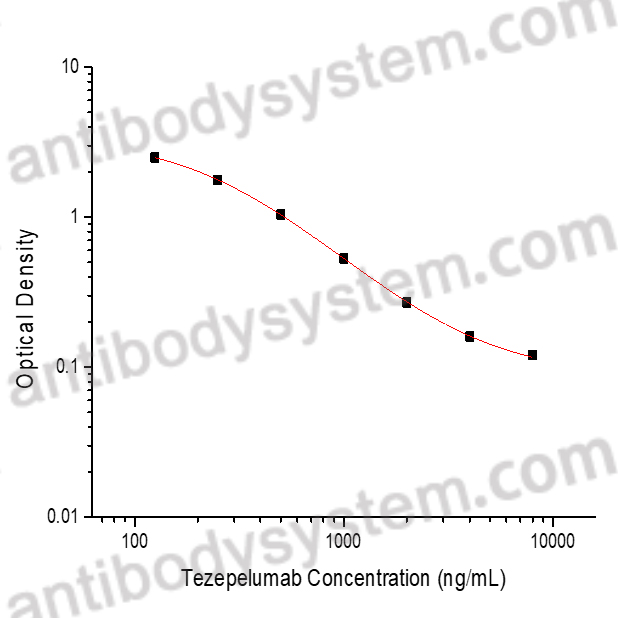
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant human TSLP has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tezepelumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tezepelumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tezepelumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Tezepelumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 8,000 ng/mL
Sensitivity
88.43 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2930.1
|
582.4
|
195.7
|
3063.1
|
570.8
|
200.2
|
|
Standard deviation
|
201.5
|
27.5
|
17.3
|
269.1
|
28.2
|
15.5
|
|
CV (%)
|
6.9
|
4.7
|
8.8
|
8.8
|
4.9
|
7.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
AMG 157, AMG-157, MEDI-9929, MEDI9929, anti-TSLP-Receptor (TSLP-R, Crl2), CAS: 1572943-04-4
Background
Tezepelumab is a first-in-class human monoclonal antibody that binds to TSLP, thus inhibiting its interaction with TSLP receptor complex. Tezepelumab given as an add-on-therapy to patients with severe uncontrolled asthma has shown safety, tolerability and efficacy. Several trials are evaluating the long-term safety and the efficacy of tezepelumab in adults and adolescents with severe uncontrolled asthma.
WAYPOINT: Are we there yet for patients with nasal polyposis?, PMID:40516522
Compassionate use of tezepelumab in near fatal asthma: A case report and review of the literature., PMID:40492166
Aspirin-Exacerbated Respiratory Disease in the era of biologics., PMID:40490219
Advancements in Biologic Therapies for Pediatric Asthma: Emerging Therapies and Future Directions., PMID:40486460
Real-life preliminary evidence for basophils as predictors of Tezepelumab response in severe asthma., PMID:40471866
Mechanisms and Treatment of Type 2 High and Low Asthma Endotypes., PMID:40462371
Tezepelumab in patients with eosinophilic granulomatosis with polyangiitis (EGPA) following suboptimal response to anti-IL5/5R therapy., PMID:40409710
Effectiveness and Safety of Tezepelumab in a Diverse Population of US Patients with Severe Asthma: Initial Results of the PASSAGE Study., PMID:40388086
Clinical and Biological Remission With Tezepelumab: The Real-World Response in Severe Uncontrolled Asthma., PMID:40365686
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Combination of Allergen-Specific Immunotherapy With Biologics in Severe Asthma: Counterintuitive or Rational?, PMID:40349962
Requalification of patients with severe asthma for biological therapy-Practical 'ReQuaBi' rate decision scheme based on the analytical model., PMID:40348613
Reappraisal of Biologic Efficacy from Phase 3 Trials in Refractory Chronic Rhinosinusitis and Nasal Polyps., PMID:40340024
Switching to tezepelumab from other biologics in patients with severe asthma: a retrospective study., PMID:40339469
Is Tezepelumab the treatment option of choice in severe allergic asthma?, PMID:40323045
Tezepelumab can Restore Normal Lung Function in Patients with Severe, Uncontrolled Asthma: Pooled Results from the PATHWAY and NAVIGATOR Studies., PMID:40285963
New Therapeutic Challenges in Pediatric Gastroenterology: A Narrative Review., PMID:40281872
Clarification of the efficacy of tezepelumab in the phase 2a COURSE trial., PMID:40266168
Efficacy of Biologics in Reducing Exacerbations Requiring Hospitalization or an Emergency Department Visit in Patients with Moderate or Severe, Uncontrolled Asthma., PMID:40261563
The impact of tezepelumab therapy on perceived asthma triggers: a multicenter real-life study., PMID:40257396
Effectiveness of tezepelumab in preventing relapse of eosinophilic granulomatosis with polyangiitis: A case report., PMID:40239417
Tezepelumab achieves improvement of severe uncontrolled asthma and rhinosinusitis: Case series., PMID:40226773
Treatment of allergic bronchopulmonary aspergillosis with biologics., PMID:40226607
Real-World Evidence of Administration of Biologic Agents in Patients with Severe Asthma: An Analysis of the Respiratory Department of University Hospital of Patras Asthma Registry., PMID:40217624
Translational Investigation of CM326 from Preclinical Studies to Randomized Phase I Clinical Trials in Healthy Adults., PMID:40185989
Efficacy of tezepelumab in patients with severe, uncontrolled asthma and aspirin-exacerbated respiratory disease in NAVIGATOR., PMID:40180239
Biologics for Chronic Rhinosinusitis With Nasal Polyps: Current Status and Clinical Considerations in Korea., PMID:40169027
Oscillometry and spirometry derived ratios to assess small airways dysfunction in severe asthma patients taking tezepelumab., PMID:40158666
Head-To-Head Comparison of Biologic Efficacy in Asthma: What Have We Learned?, PMID:40156481
Severe Asthma and Active SARS-CoV-2 Infection: Insights into Biologics., PMID:40149651
A real-world study on tezepelumab effectiveness in severe asthma focusing on small airway dysfunction., PMID:40147569
Worse airflow obstruction but not type 2 biomarkers identifies super-responders to tezepelumab in real life., PMID:40139437
Clinical efficacy and mechanisms of biologics for chronic rhinosinusitis with nasal polyps., PMID:40132672
Tezepelumab in Adults with Severe Chronic Rhinosinusitis with Nasal Polyps., PMID:40106374
Adverse effects of biologics used to treat asthma., PMID:40099886
Real-life effectiveness of tezepelumab in severe asthma., PMID:40088034
Tezepelumab inhibits highly functional truncated thymic stromal lymphopoietin in chronic rhinosinusitis., PMID:40057283
Successful Treatment of Chronic Rhinosinusitis With Nasal Polyps (CRSwNP) With Tezepelumab: A Case Report., PMID:40024624
Tezepelumab for asthma with current or previous smoking habit: Case series., PMID:40008096
Severe Asthma in School-Age Children: An Updated Appraisal on Biological Options and Challenges in This Age Group., PMID:40003269
Tailoring Biologic Therapies for Pediatric Severe Asthma: A Comprehensive Approach., PMID:40003242
Update in paediatric asthma., PMID:39973758
Tezepelumab for the treatment of chronic spontaneous urticaria: Results of the phase 2b INCEPTION study., PMID:39956278
Response and remission in asthma with tezepelumab: overlapping concepts informing on type-2 inflammatory-dependent treatment effects., PMID:39947688
Reply to: Response and remission in asthma with tezepelumab: overlapping concepts informing on type-2 inflammatory-dependent treatment effects., PMID:39947687
Three-dimensional bronchial tree visualization in exercise-induced severe asthma following tezepelumab treatment., PMID:39896213
Tezepelumab treatment in severe asthma with recurrent chronic rhinosinusitis with nasal polyps: Case series., PMID:39896212
Biologic Therapies for Chronic Obstructive Pulmonary Disease: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials., PMID:39877958
Real-world effects of tezepelumab on small airway dysfunction in severe refractory asthma., PMID:39870209
Targeting alarmins in asthma: From bench to clinic., PMID:39855362