Catalog No.
KDJ04003
Description
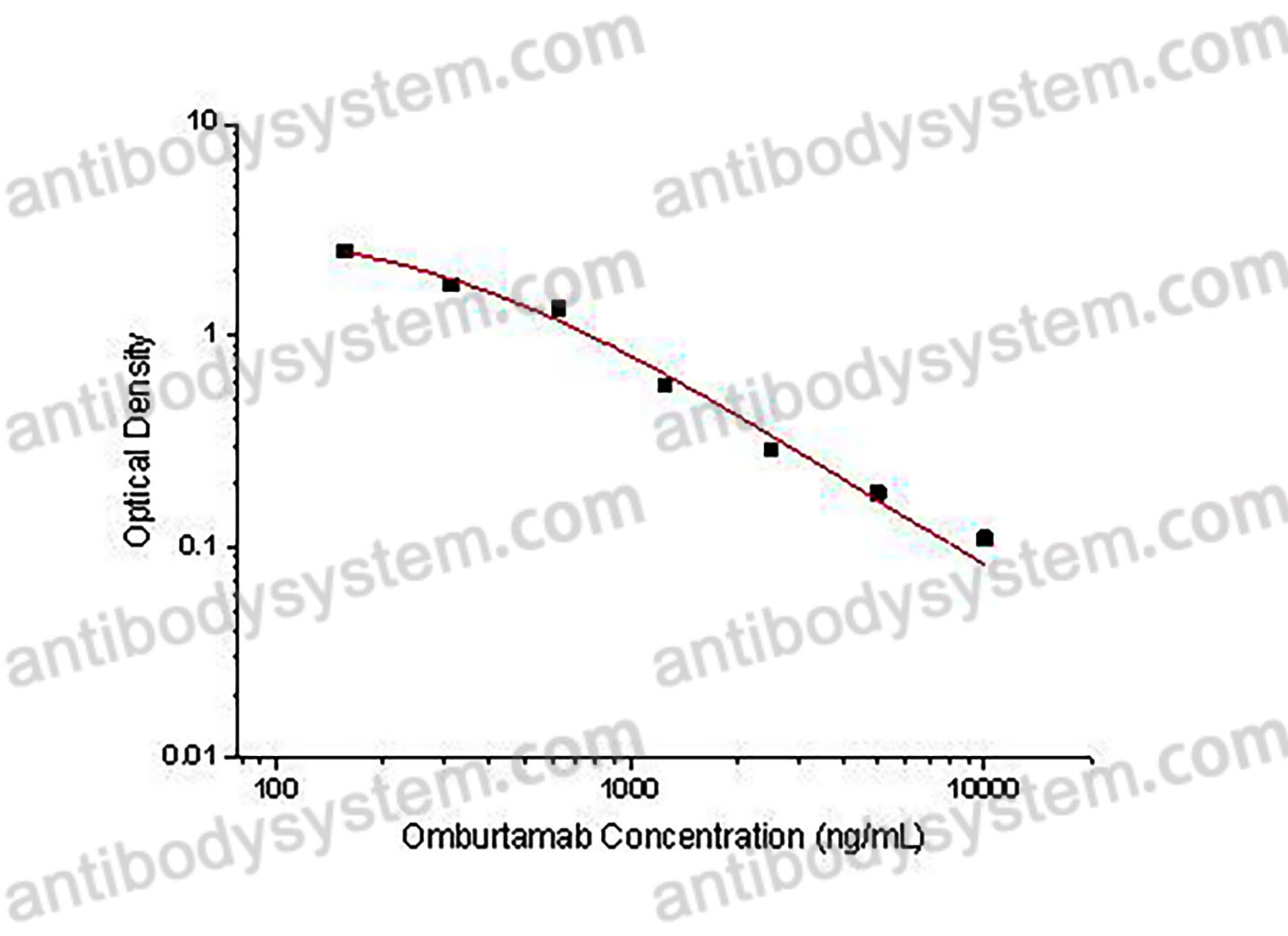
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD276 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Omburtamab in the sample competitively binds to the pre-coated protein with biotin-labeled Omburtamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Omburtamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Omburtamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
108.57 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
6034.9
|
1301.3
|
277.9
|
4865.2
|
1240.8
|
317.4
|
|
Standard deviation
|
332.7
|
96.9
|
27.2
|
377.0
|
86.1
|
39.5
|
|
CV (%)
|
5.5
|
7.4
|
9.8
|
7.7
|
6.9
|
12.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
8H9, Mab 8H9, CAS: 1895083-75-6
Erratum to: Phase 1 dose-escalation trial using convection-enhanced delivery of radio-immunotheranostic 124I-Omburtamab for diffuse intrinsic pontine glioma., PMID:40489365
Determination of the Intralesional Distribution of Theranostic 124I-Omburtamab Convection-Enhanced Delivery in Treatment of Diffuse Intrinsic Pontine Glioma., PMID:40306974
Phase 1 dose-escalation trial using convection-enhanced delivery (CED) of radio-immunotheranostic 124I-Omburtamab for diffuse intrinsic pontine glioma (DIPG)., PMID:39969230
Theranostic Intratumoral Convection-Enhanced Delivery of 124I-Omburtamab in Patients with Diffuse Intrinsic Pontine Glioma: Pharmacokinetics and Lesion Dosimetry., PMID:39142829
Feasibility of safe outpatient treatment in pediatric patients following intraventricular radioimmunotherapy with 131I-omburtamab for leptomeningeal disease., PMID:39083105
Feasibility of safe outpatient treatment in pediatric patients following intraventricular radioimmunotherapy with 131I-omburtamab for leptomeningeal disease., PMID:38464207
B7-H3 Inhibitors in Oncology Clinical Trials: A Review., PMID:38327753
Outcomes of intraventricular 131-I-omburtamab and external beam radiotherapy in patients with recurrent medulloblastoma and ependymoma., PMID:36853490
Radioimmunoscintigraphy and Pretreatment Dosimetry of 131I-Omburtamab for Planning Treatment of Leptomeningeal Disease., PMID:36759197
Phase 1 study of intraventricular 131I-omburtamab targeting B7H3 (CD276)-expressing CNS malignancies., PMID:36371226
Biodistribution and Radiation Dosimetry of Intraperitoneally Administered 124I-Omburtamab in Patients with Desmoplastic Small Round Cell Tumors., PMID:34857661
Effective killing of cells expressing CD276 (B7-H3) by a bispecific T cell engager based on a new fully human antibody., PMID:34601396
Evaluation of a patient-specific algorithm for predicting distribution for convection-enhanced drug delivery into the brainstem of patients with diffuse intrinsic pontine glioma., PMID:33990084
Mast cell proliferation in the cerebrospinal fluid after intraventricular administration of anti-B7H3 immunotherapy., PMID:33533945
Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes., PMID:33514401
B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study., PMID:33119478
IntraOmmaya compartmental radioimmunotherapy using 131I-omburtamab-pharmacokinetic modeling to optimize therapeutic index., PMID:33047248
Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma., PMID:32977309
Antibodies to watch in 2020., PMID:31847708
Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine., PMID:31481583
Biodistribution and Dosimetry of Intraventricularly Administered 124I-Omburtamab in Patients with Metastatic Leptomeningeal Tumors., PMID:31405921
Targeted radioimmunotherapy for embryonal tumor with multilayered rosettes., PMID:30879172
Antibodies to watch in 2019., PMID:30516432