Catalog No.
KDH72401
Description
PRINCIPLE OF THE ASSAY
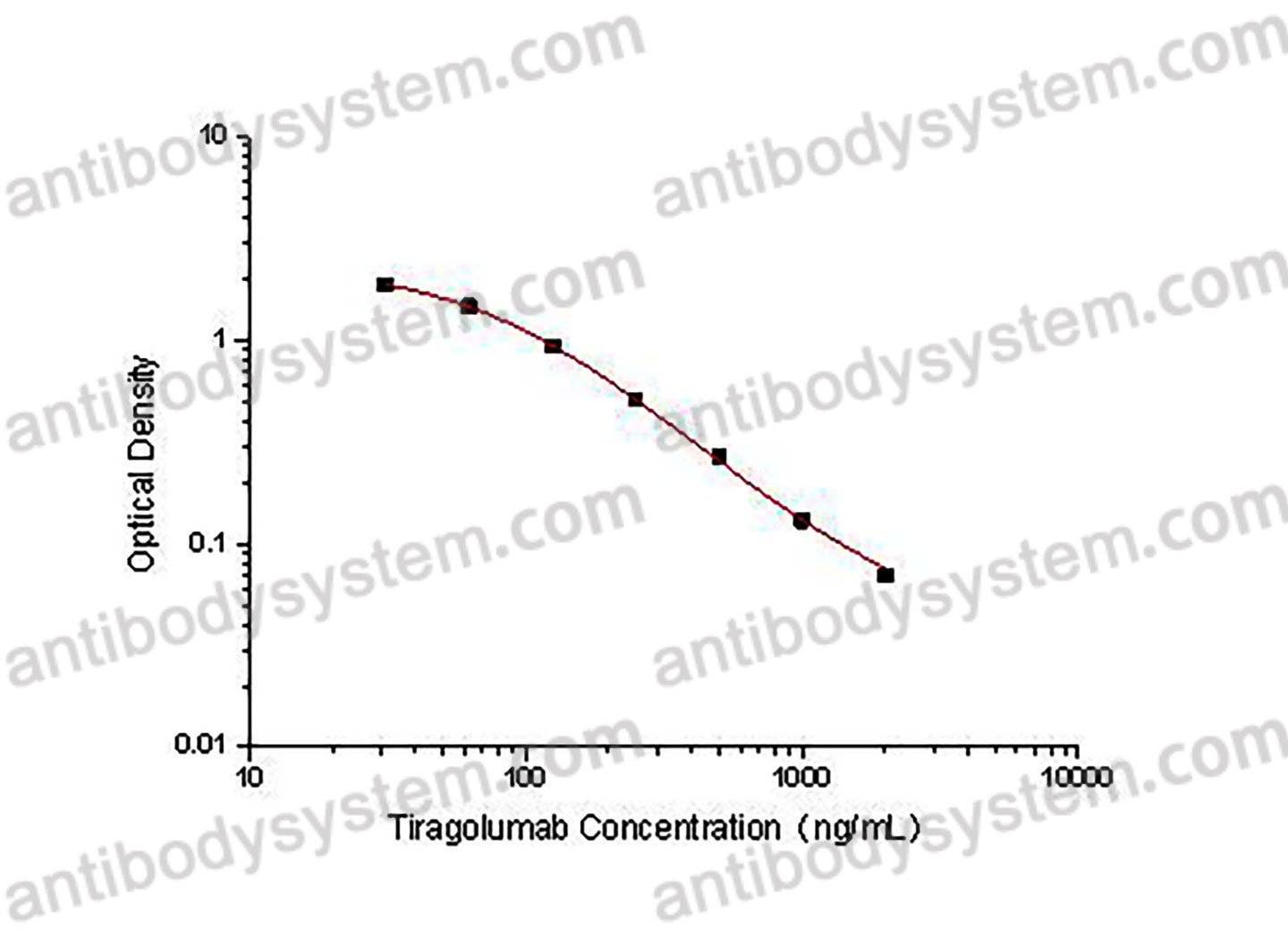
This assay employs the quantitative competitive enzyme immunoassay technique. A recombinant human TIGIT antigen has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tiragolumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tiragolumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tiragolumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Tiragolumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
21.35 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
941.1
|
249.3
|
58.9
|
944.2
|
236.0
|
57.5
|
|
Standard deviation
|
46.3
|
7.7
|
5.1
|
67.1
|
13.2
|
6.5
|
|
CV (%)
|
4.9
|
3.1
|
8.6
|
7.1
|
5.6
|
11.3
|
Recovery
75-110%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
MTIG-7192-A, MTIG-7192A, MTIG7192A, RG-6058, RO-7092284, RO7092284, CAS: 1918185-84-8
Background
Tiragolumab is an investigational, fully human monoclonal IgG1 antibody designed to prevent PVR-TIGIT interaction.
Unlocking the potential of TIGIT in enhancing therapeutic strategies for acute myeloid leukemia through combined azacitidine therapy., PMID:40374899
NeoTRACK trial: Neoadjuvant TiRagolumab, Atezolizumab and Chemotherapy - dissection of IO- efficacy in NSCLC by longitudinal tracKing - protocol of a non-randomised, open-label, single-arm, phase II study., PMID:40147985
Staphylococcus aureus-specific TIGIT+ Treg are present in the blood of healthy subjects - a hurdle for vaccination?, PMID:39981298
Model-Informed Selection of the Recommended Phase 2 Dosage for Anti-TIGIT Immunotherapy Leveraging co-Expressed PD-1 Inhibitor Target Engagement., PMID:39921877
Tiragolumab in combination with atezolizumab and bevacizumab in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (MORPHEUS-Liver): a randomised, open-label, phase 1b-2, study., PMID:39855251
Development and characterisation of [18F]TTDP, a novel T cell immunoglobulin and ITIM domain tracer, in humanised mice and non-human primates., PMID:39297961
The efficacy and safety of immunotherapy as first-line treatment for extensive-stage small cell lung cancer: evaluating based on reconstructed individual patient data., PMID:39026980
IMbrave152/SKYSCRAPER-14: a Phase III study of atezolizumab, bevacizumab and tiragolumab in advanced hepatocellular carcinoma., PMID:38861301
A non-comparative, randomized, phase II trial of atezolizumab or atezolizumab plus tiragolumab for programmed death-ligand 1-positive recurrent cervical cancer (SKYSCRAPER-04)., PMID:38858106
Implementation of a Three-Way Comparability Assessment for a Bioanalytical Anti-Drug Antibody Method., PMID:38637446
Efficacy and safety of novel immune checkpoint inhibitor-based combinations versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer: A network meta-analysis., PMID:38623838
Rethinking strategies in SCLC: Lessons learned from tiragolumab in the SKYSCRAPER-02 study., PMID:38614072
Correction to: Phase I study of the anti‑TIGIT antibody tiragolumab in combination with atezolizumab in Japanese patients with advanced or metastatic solid tumors., PMID:38556530
Structural insights into the unique pH-responsive characteristics of the anti-TIGIT therapeutic antibody Ociperlimab., PMID:38460520
Phase I pharmacokinetic, safety, and preliminary efficacy study of tiragolumab in combination with atezolizumab in Chinese patients with advanced solid tumors., PMID:38451273
Anti-TIGIT antibody improves PD-L1 blockade through myeloid and Treg cells., PMID:38418879
Targeting TIGIT for cancer immunotherapy: recent advances and future directions., PMID:38229100
Phase I study of the anti-TIGIT antibody tiragolumab in combination with atezolizumab in Japanese patients with advanced or metastatic solid tumors., PMID:38206370
Pharmacokinetics (PK) of Tiragolumab in First-in-Human Study in Patients with Mixed Solid Tumors (GO30103)., PMID:38105505
SKYSCRAPER-02: Tiragolumab in Combination With Atezolizumab Plus Chemotherapy in Untreated Extensive-Stage Small-Cell Lung Cancer., PMID:37976444
A phase I study of the combination of atezolizumab, tiragolumab, and stereotactic body radiation therapy in patients with metastatic multiorgan cancer., PMID:37946136
Anti-TIGIT Antibody Tiragolumab Alone or With Atezolizumab in Patients With Advanced Solid Tumors: A Phase 1a/1b Nonrandomized Controlled Trial., PMID:37768658
Acute liver failure following immune checkpoint inhibitors., PMID:37660741
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis., PMID:37435087
Anti-TIGIT therapies for solid tumors: a systematic review., PMID:36933320
Tiragolumab (Anti-TIGIT) in SCLC: Skyscraper-02, a Towering Inferno., PMID:36636263
Tiragolumab and atezolizumab in patients with PD-L1 positive non-small-cell lung cancer., PMID:35654050
Tiragolumab Results Cast Shadow on TIGIT Pipeline., PMID:35652612
Tiragolumab active in PD-L1+ NSCLC., PMID:35606416
Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study., PMID:35576957
Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer., PMID:35292828
TIGIT/CD226 Axis Regulates Anti-Tumor Immunity., PMID:33670993
ASCO 2020 non-small lung cancer (NSCLC) personal highlights., PMID:33456617
Advances in the management of non-small cell lung cancer (NSCLC): A new practice changing data from asco 2020 annual meeting., PMID:33271494
Tiragolumab Impresses in Multiple Trials., PMID:32576590