Catalog No.
KDH28807
Description
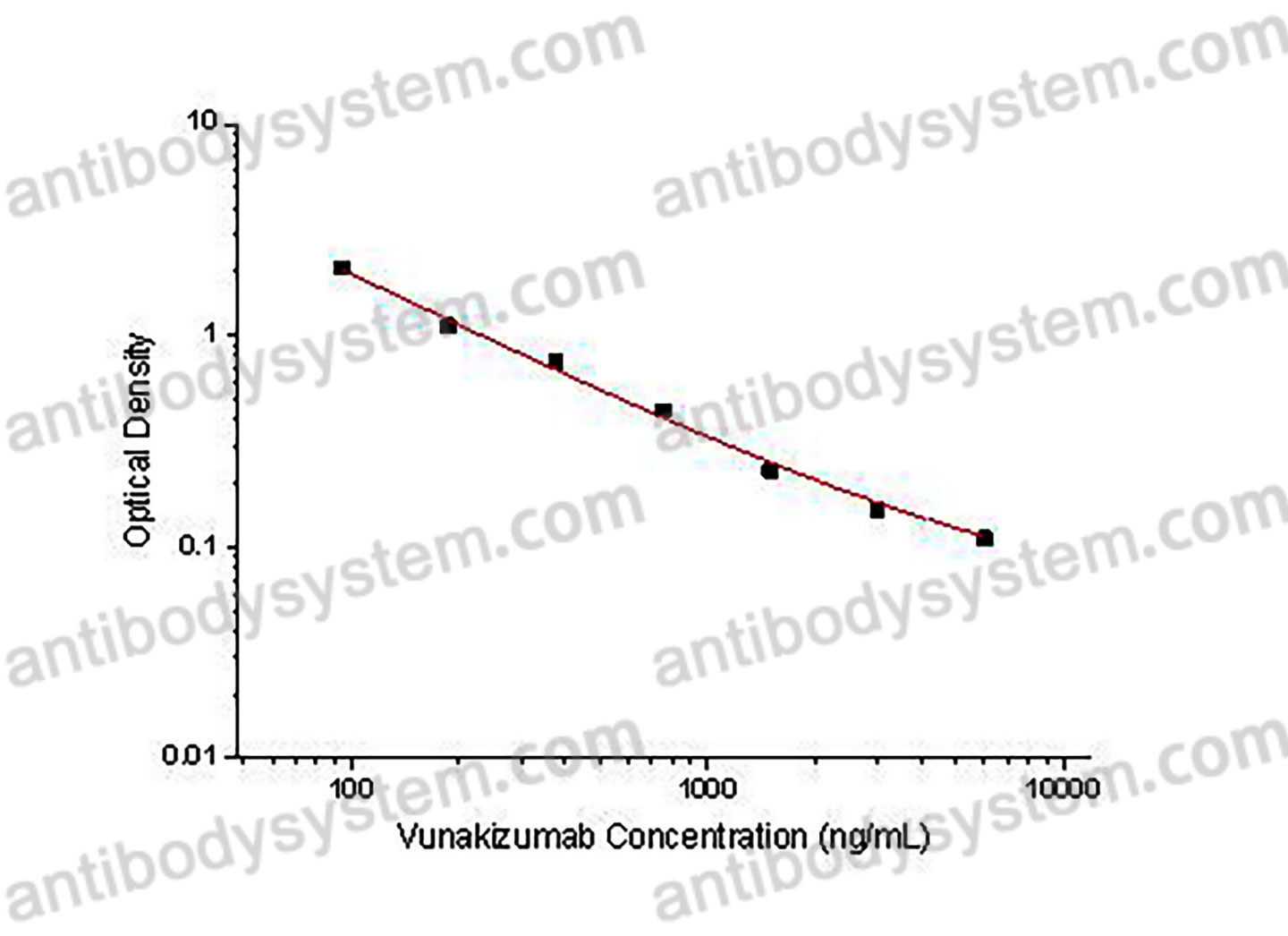
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL17A has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Vunakizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Vunakizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Vunakizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Vunakizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
93.75 - 6,000 ng/mL
Sensitivity
51.46 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
2301.8 |
675.1 |
172.0 |
4753.4 |
640.8 |
138.9 |
|
Standard deviation |
208.2 |
69.5 |
15.9 |
654.7 |
80.9 |
22.2 |
|
CV (%) |
9.0 |
10.3 |
9.2 |
13.8 |
12.6 |
16.0 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
SHR-1314, CAS: 1792181-33-9
Background
Vunakizumab is a recombinant humanized IgGκ monoclonal antibody binds to and neutralises the proinflammatory cytokine interleukin-17A (IL-17A). It was discovered and developed by Jiangsu Hengrui Medicine (China) with the developmental name SHR-1314, for the treatment of autoimmune diseases such as psoriasis, psoriatic arthritis, ankylosing spondylitis, multiple sclerosis and inflammatory arthritis. Atridia Pty. Ltd., which is now a part of Jiangsu Hengrui Medicine, start a phase-I clinical trial in psoriasis (in volunteers) in Australia in August 2016. Atridia completed the phase-I trial in July 2017. A phase-I/II study, which is started from December 2017, is ongoing to assess the safety, tolerability, and pharmacokinetics of SHR-1314 with expanded dose finding in subjects with moderate-to-severe plaque psoriasis.