Catalog No.
KDH28805
Description
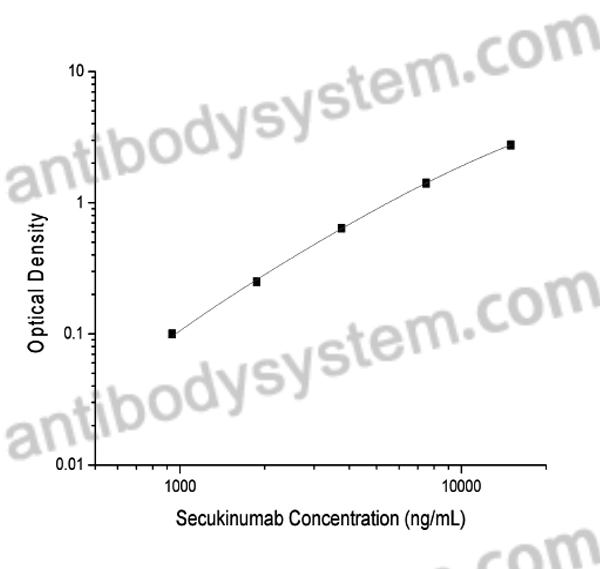
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human IL17A has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Secukinumab present is bound by the immobilized protein. After washing away any unbound substances, abiotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Secukinumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Secukinumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
937.5 - 15,000 ng/mL
Sensitivity
715.94 ng/mL.
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
7621.5
|
1720.7
|
952.0
|
6774.0
|
1688.3
|
1001.7
|
|
Standard deviation
|
264.2
|
60.8
|
42.0
|
372.9
|
134.7
|
65.9
|
|
CV (%)
|
3.5
|
3.5
|
4.4
|
5.5
|
8.0
|
6.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
AIN457, CAS: 1229022-83-6
Background
Secukinumab (Cosentyx) is a fully human IgG1/κ-class monoclonal antibody (mAb) composed of 215 light chain amino acids and 457 heavy chain amino acids. It was discovered and developed by Novartis with the developmental name AIN457, for the treatment of uveitis, rheumatoid arthritis, ankylosing spondylitis, and psoriasis. It is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology.
Dose modulation strategies in psoriatic patients: real-world comparison between secukinumab and brodalumab for up to one year after dose spacing., PMID:40499920
Deucravacitinib as a monotherapy for concurrent management of psoriasis and chronic spontaneous urticaria., PMID:40490621
Health Equity and Secukinumab for Severe Psoriasis in New Zealand., PMID:40488606
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
EXPRESS: THE FREQUENCY, PREDICTORS AND TREATMENT OPTIONS OF SPONDYLOARTHRITIS IN PRIMARY SJÖGREN'S SYNDROME: A SINGLE-CENTER EXPERIENCE., PMID:40488278
Uveitis in patients with axial spondyloarthritis or psoriatic arthritis: a post hoc analysis from placebo-controlled phase III studies with secukinumab., PMID:40487540
Effects of secukinumab on skeletal microarchitecture and vertebral fractures in patients with axial spondyloarthritis using HR-pQCT., PMID:40481972
Case Report: Effectiveness of deucravacitinib in chronic recurrent multifocal osteomyelitis and concomitant psoriasis., PMID:40475785
Physiologically Based Pharmacokinetic/Pharmacodynamic Model for Secukinumab: Dose Exploration in Pediatric Patients with Plaque Psoriasis., PMID:40464650
Safety and efficacy of IL-17 inhibitors in patients with comorbid multiple sclerosis/multiple Sclerosis-like syndrome: a systematic review., PMID:40459586
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
IL-17A inhibitor-induced ulcerative colitis treated with an anti-IL-23 antibody., PMID:40455166
Model-Informed Drug Development-Based Bridging from Subcutaneous to Intravenous Secukinumab Dosing: Approval in Psoriatic Arthritis and Axial Spondyloarthritis., PMID:40454543
A Matching-Adjusted Indirect Comparison of Guselkumab and Secukinumab in Patients with Psoriatic Arthritis Over 52 Weeks., PMID:40450642
Case Report: Successful Treatment of Secukinumab-Induced SAPHO Syndrome With Tofacitinib., PMID:40443046
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Clinical Characteristics of CARD14-Associated Papulosquamous Eruption and Evaluation of Therapeutic Efficacy of Secukinumab., PMID:40433052
Evaluation of Combination Therapy With Secukinumab and Systemic Corticosteroids for Acute Generalized Exanthematous Pustulosis : A Retrospective Cohort Study., PMID:40419223
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Causes of drug-induced photosensitivity: an analysis using FDA adverse event reporting system database., PMID:40413262
Real-World Utilization of Biologic and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis: Insights from Sweden and Germany., PMID:40402376
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Biologic and Non-Biologic Therapies for Scalp Psoriasis: A Network Meta-analysis of Randomized Controlled Trials., PMID:40401874
Safety and Efficacy of Secukinumab in a Child with CARD14-Associated Papulosquamous Eruption: A Case Report and Review of Literature., PMID:40395577
Systemic secukinumab treatment in patients with ankylosing spondylitis: the relationship between systemic and tear proinflammatory cytokines and ocular surface findings-a pilot study., PMID:40394377
Bayesian trial of adalimumab versus secukinumab for children with juvenile idiopathic arthritis associated uveitis or chronic anterior uveitis., PMID:40390069
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Access, Effectiveness, Safety And Survival Of Secukinumab In Patients With Axial Spondyloarthritis And Axial Psoriatic Arthritis: Real-Life Study In Argentina., PMID:40374511
Biologic therapies and small molecules in the treatment of hidradenitis suppurativa., PMID:40373949
Assessing Long-Term Pain Reduction with Secukinumab in Moderate to Severe Hidradenitis Suppurativa: A Post Hoc Analysis of the SUNSHINE and SUNRISE Phase 3 Trials., PMID:40372667
Extension of Secukinumab and Ixekizumab Dose for Moderate-To-Severe Psoriasis in Low Disease Activity Intervals., PMID:40369849
Secukinumab is effective and safe for patients with giant cell arteritis after tocilizumab failure., PMID:40366735
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Combined biological therapy with dupilumab and secukinumab for coexisting bullous pemphigoid and psoriasis., PMID:40357969
Secukinumab induced paradoxical worsening of mucocutaneous features of Behcet's disease., PMID:40357952
Apremilast Coadministered with Secukinumab for Effective Treatment of Acrodermatitis Continua of Hallopeau: A Case Report., PMID:40351850
Hypoxia-induced RHCG as a key regulator in psoriasis and its modulation by secukinumab., PMID:40351602
First case report of oral histoplasmosis in a patient receiving Secukinumab (Cosentyx): opportunistic infections as complication of emerging immunomodulatory therapies., PMID:40350329
Treatment of severe psoriasis in a hospice care patient using secukinumab, an inhibitor of interleukin-17A expression: Treatment response and changes in quality of life., PMID:40329840
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Letter to the Editor for "Which Is the Best Option for AxSpA Patients After First TNFi Failure: Switch to Secukinumab or Cycling With Other TNFi?"., PMID:40312866
Secukinumab Leading to Rapid Improvement in Pyogenic Arthritis, Acne, Pyoderma Gangrenosum, and Hidradenitis Suppurativa (PAPASH) Syndrome: A Case Report and Review of Treatment Modalities for PAPASH Patients., PMID:40309341
Secukinumab as a Novel Treatment for Chronic Netherton Syndrome in a Young Adult., PMID:40299794
Novel Small-Molecule Treatment and Emerging Biological Therapy for Psoriasis., PMID:40299379
Severe psoriasis vulgaris complicating pemphigus vulgaris: A case report., PMID:40295230
Dual Biologic Therapy for Psoriasis in a Patient with Atopic Dermatitis., PMID:40290565