Catalog No.
KDH28803
Description
PRINCIPLE OF THE ASSAY
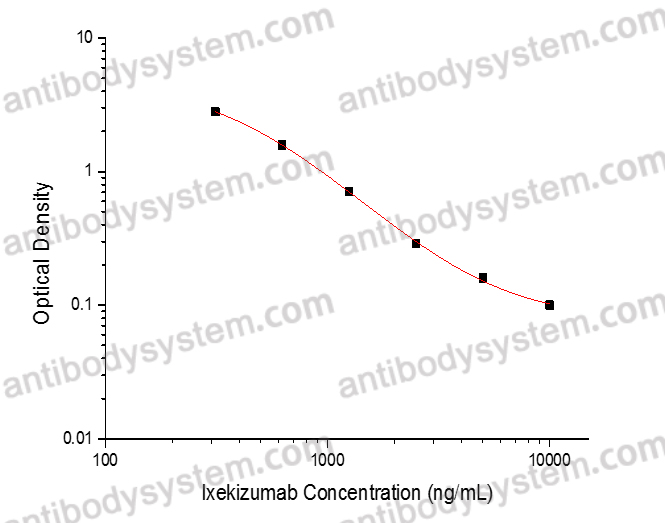
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL17A has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Ixekizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Ixekizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Ixekizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Ixekizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
78.13 - 5,000 ng/mL
Sensitivity
39.3 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
LY2439821, CAS: 1143503-69-8
Background
Ixekizumab is a humanized monoclonal antibody to interleukin-17A which acts as an antiinflammatory agent and is used to treat moderate-to-severe plaque psoriasis. Ixekizumab has not been linked to serum enzyme elevations during therapy or to instances of idiosyncratic acute liver injury.
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Understanding Psoriasis Patient Preferences for Biologic Dosing Frequencies: Insights From a Patient Survey., PMID:40476147
A Case of Erythrodermic Psoriasis Successfully Treated With Ixekizumab Combined With Low-Dose Methotrexate to Ensure Sustained Clearance: A Case Report., PMID:40475334
Pityriasis rubra pilaris with symmetric polyarthritis and lymphadenopathy., PMID:40467080
Safety and efficacy of IL-17 inhibitors in patients with comorbid multiple sclerosis/multiple Sclerosis-like syndrome: a systematic review., PMID:40459586
Dissecting Cellulitis of the Scalp Successfully Treated with a Combination of Ixekizumab and Tofacitinib., PMID:40458693
The effect of IL-17 and IL-23 ınhibitors on hematological ınflammatory parameters in patients with psoriasis vulgaris., PMID:40455345
Psoriasis complicated with polymyositis successfully treated with Ixekizumab: A case report., PMID:40441209
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Paradoxical development of prurigo nodularis during ixekizumab therapy in a patient with generalized pustular psoriasis and psoriatic arthritis., PMID:40417893
Real-world safety assessment of Ixekizumab based on the FDA Adverse Event Reporting System (FAERS)., PMID:40408459
Real-World Utilization of Biologic and Targeted Synthetic Disease-Modifying Anti-rheumatic Drugs in Psoriatic Arthritis and Axial Spondyloarthritis: Insights from Sweden and Germany., PMID:40402376
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
Ixekizumab Retention Rate and Predictors of Treatment Persistence in Psoriatic Arthritis: Results of an Italian Multicenter Study., PMID:40376617
Extension of Secukinumab and Ixekizumab Dose for Moderate-To-Severe Psoriasis in Low Disease Activity Intervals., PMID:40369849
Thymic Bmi-1 hampers γδT17 generation and its derived RORγt-IL-17A signaling to delay cardiac aging., PMID:40366697
Efficacy of Ixekizumab in Chinese Patients with Radiographic Axial Spondyloarthritis by Baseline C-Reactive Protein Level., PMID:40343690
Trauma-Induced Psoriatic Arthritis: A Deep Köbner Phenomenon., PMID:40342834
Alopecia Areata Observed in a Patient Receiving Ixekizumab: A Case Report., PMID:40341668
Immunoproteomic Response to IL-17A Blockade in a Patient With Generalized Pustular Psoriasis: New Insights From Plasma Olink Profiling., PMID:40326672
Emerging manifestations of IL-17 immunomodulation in the gastrointestinal tract., PMID:40319948
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Persistence of ixekizumab in psoriatic arthritis and the influence of gender., PMID:40315011
Ixekizumab demonstrates targeted protection against psoriatic arthritis in psoriasis patients: A multicenter retrospective cohort analysis., PMID:40288535
Efficacy and Safety of IL-17 and IL-23 Inhibitors in Elderly Patients With Plaque Psoriasis: A Real-World Study., PMID:40285439
Targeting nail psoriasis: IL-17A inhibitors demonstrate site-specific superiority over IL-23 inhibitor in a 24-week dermoscopy-guided real-world cohort., PMID:40264783
Case Report: Spesolimab for pyoderma gangrenosum in an undifferentiated oligoarthritis patient receiving anti-IL-17 therapy., PMID:40260260
[Successful subcutaneous desensitization in a patient with systemic reaction due to ixekizumab]., PMID:40253637
Unveiling the effectiveness and safety spectrum of biologic therapies in psoriasis: a three-year real-world analysis., PMID:40227059
BASDAI and ASDAS disease states in relationship to ASAS40 response: post hoc analysis of ixekizumab in radiographic axial spondyloarthritis., PMID:40201598
Chinese herbal medicine (Guben Qushi Huayu formula) combined with Ixekizumab in reducing psoriasis vulgaris relapse: Protocol for a mixed-methods research study., PMID:40191426
Treatment of Psoriasis with II-17 Inhibitors: Comparison of Long-Term Effectiveness and Drug Survival of Secukinumab vs Ixekizumab in Real-World Practice., PMID:40166484
The Efficacy, Safety and Longevity of Biologic Treatments in Pediatric and Adult Psoriasis Patients: A Comparative Multi-Center, Real-Life Study., PMID:40165569
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Small molecule interleukin (IL) 17A/A antagonists and antibodies blocking both IL17A/A and IL17A/F demonstrate equivalent degrees of efficacy in preclinical models of skin and joint inflammation., PMID:40127522
Hypopigmented mycosis fungoides-like eruption following ixekizumab treatment., PMID:40123792
A real-world study of ixekizumab use patterns, switching and efficacy in patients with plaque psoriasis., PMID:40119943
Risk of Herpes Zoster and Postherpetic Neuralgia in Patients with Psoriasis Treated with Biologics: A nationwide study using target trial emulation framework., PMID:40112174
Intra-Class Interleukin-(IL)-17 Blocker Switching: Ixekizumab as a Solution for Secukinumab Non-responders in Secondary Biologic Failure in Psoriasis., PMID:40092026
Safety and effectiveness of ixekizumab in Japanese patients with psoriasis vulgaris, psoriatic arthritis, generalized pustular psoriasis, and erythrodermic psoriasis: Post-marketing surveillance., PMID:40079483
A systematic review of the role of interleukin-17 inhibitors in bullous pemphigoid: therapeutic and paradoxical effects., PMID:40067544
Pityriasis Rubra Pilaris Following COVID-19 Infection: A Case of Successful Treatment With Ixekizumab., PMID:40062179
New and future perspectives in Behçet's syndrome., PMID:40060132
Baseline Pathological Liver Function Tests in Patients With Psoriasis Support the Indication for Systemic Therapy Rather Than Being a Reason Against It: A Real-World Analysis., PMID:40046951
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Acute Generalized Exanthematous Pustulosis in a 76-year-Old Man With Neuroendocrine Carcinoma of the Lung, Responsive to Ixekizumab., PMID:40026376
Safety of Interleukin Inhibitors in Psoriatic Patients with Latent Tuberculosis Infection Without Chemoprophylaxis: A Systematic Review., PMID:40026108
Eczematous eruption after ixekizumab successfully treated with upadacitinib., PMID:40026044
Synergistic Improvements in Synovitis, Enthesitis, and Patient-Reported Outcomes for Patients with Psoriatic Arthritis Treated with Ixekizumab in SPIRIT Trials., PMID:40014255