Catalog No.
KDH02207
Description
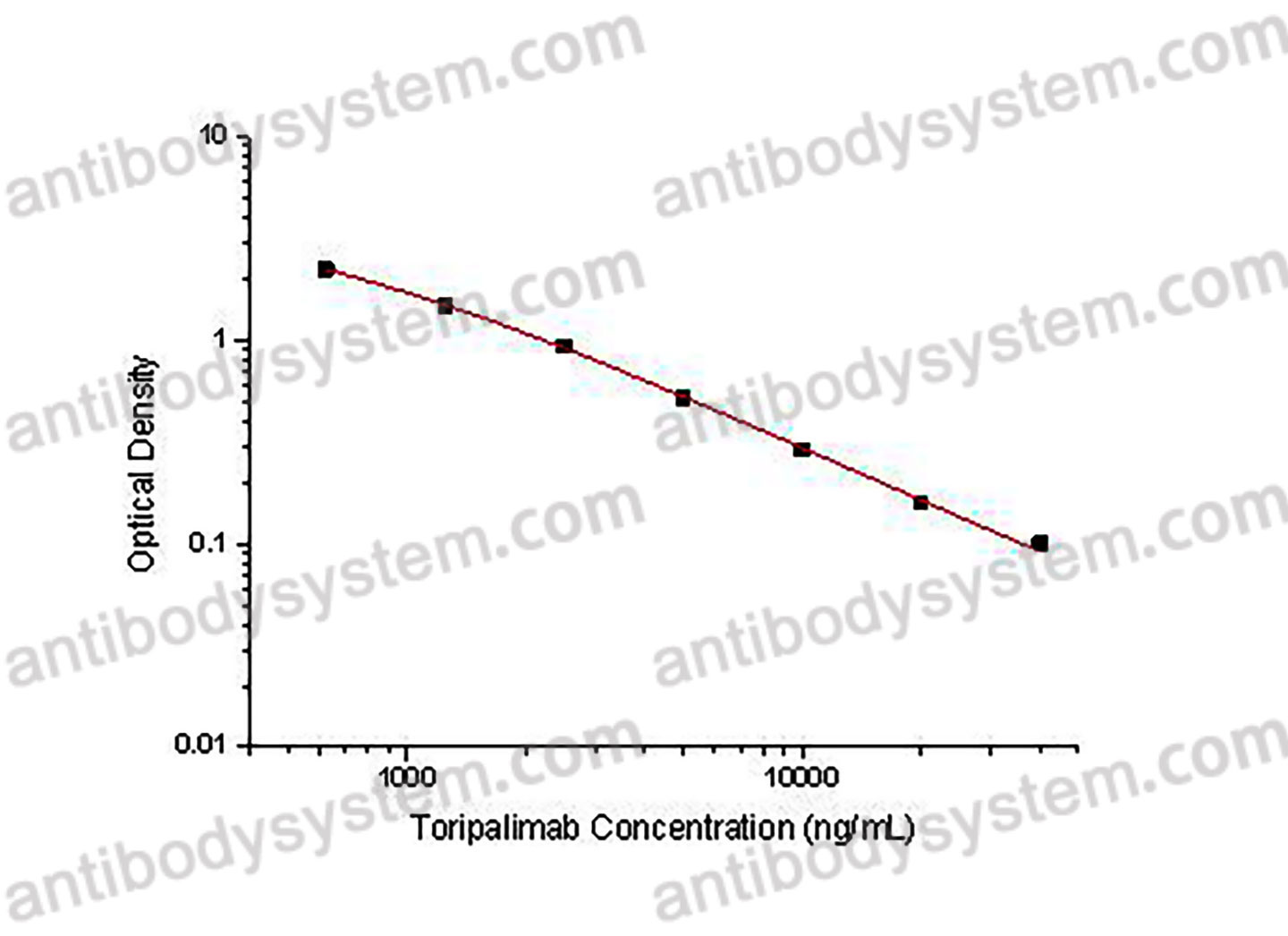
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD279 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Toripalimab in the sample competitively binds to the pre-coated protein with biotin-labeled Toripalimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Toripalimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Toripalimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
625 - 40,000 ng/mL
Sensitivity
414.85 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
38229.5
|
8558.4
|
1763.8
|
38995.6
|
8232.6
|
1622.2
|
|
Standard deviation
|
3558.2
|
383.2
|
103.7
|
6903.1
|
860.2
|
155.7
|
|
CV (%)
|
9.3
|
4.5
|
5.9
|
17.7
|
10.4
|
9.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CAS: 1924598-82-2
Background
Toripalimab is an anti-PD-1 monoclonal antibody developed for its ability to block PD-1 interactions with its ligands, PD-L1 and PD-L2, and for enhanced receptor endocytosis function. PD-L1 and PD-L2 are tumor checkpoint proteins which recognize the PD-1 receptor on T cells and exhaust their anti-tumor activity. Blocking PD-1 interactions with PD-L1 and PD-L2 is thought to recharge the immune system’s ability to attack and kill tumor cells.
GFH018 and toripalimab combination therapy for previously treated recurrent or metastatic nasopharyngeal carcinoma: Results from a phase 1b/2 study., PMID:40512241
Efficacy and safety of SABR/partial-SABR combined with axitinib and toripalimab in recurrent or metastatic renal cell carcinoma: Preliminary results from a prospective phase 2 trial., PMID:40503038
First-line toripalimab plus chemotherapy versus chemotherapy for advanced esophageal squamous cell carcinoma: A cost-effectiveness analysis., PMID:40493606
Case Report: Remarkable and sustained remission of an anaplastic thyroid carcinoma patient to the combined treatment of multimodal radiotherapy, anlotinib and toripalimab., PMID:40469189
First-line treatment with a combination of immunotherapy, anti-EGFR monoclonal antibodies, and chemotherapeutics for unresectable left KRAS/BRAF wild-type microsatellite-stable colorectal cancer: a case report., PMID:40460045
Neoadjuvant toripalimab combined with chemotherapy in locally advanced HNSCC., PMID:40458723
Cost-effectiveness analysis of Toripalimab regimen for extensive-stage small-cell lung cancer in China and America., PMID:40453073
Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial., PMID:40450051
Primary adenocarcinoma of the renal pelvis: A case report., PMID:40441222
Phase 1b/2 study of ADG106, a 4-1BB/CD137 agonist, in combination with toripalimab in patients with advanced solid tumors., PMID:40421181
Efficacy of toripalimab in combination with anlotinib in recurrent undifferentiated pleomorphic sarcoma of the sinonasal region: a case report with biomarker analysis., PMID:40416963
Toripalimab plus bevacizumab versus sorafenib as first-line treatment for advanced hepatocellular carcinoma (HEPATORCH): a randomised, open-label, phase 3 trial., PMID:40409323
Tifcemalimab as monotherapy or in combination with toripalimab in patients with relapsed/refractory lymphoma: a Phase I trial., PMID:40379637
Phase I/II Study of Tifcemalimab, an Anti-B and T-lymphocyte Attenuator Antibody, in Combination with Toripalimab in Previously Treated Advanced Lung Cancer., PMID:40378060
Porustobart (HBM4003) plus toripalimab as second-line therapy in patients with advanced hepatocellular carcinoma: a multicenter, open-label, phase I study., PMID:40378055
Case Report: Combined PD-1 and tyrosine kinase blockade stabilizes refractory pancreatic cancer guided by the spatial structure of tumor immune microenvironment., PMID:40376004
Toripalimab-Associated Arthritis and Peripheral Neuropathy., PMID:40366363
Evaluating the efficacy and safety of bladder-sparing regimen with Disitamab Vedotin combined with Toripalimab and pelvic lymph node dissection in muscle-invasive bladder cancer patients: study protocol of a multicenter single-arm phase II trial., PMID:40361038
Clinical manifestations and risk factors of immune-related thyroid adverse events in patients treated with PD-1 inhibitors: a case-control study., PMID:40356893
Aggressive recurrent intestinal-type adenocarcinoma of the nasal cavity: a case report and literature review., PMID:40303364
Diagnosis and treatment of pulmonary lymphoepithelioma-like carcinoma: A case report., PMID:40290685
Perioperative the BTLA inhibitor (tifcemalimab) combined with toripalimab and chemotherapy for resectable locally advanced thoracic esophageal squamous cell carcinoma trial (BT-NICE trial): a prospective, single-arm, exploratory study., PMID:40276504
Both complete response and long-term survival after combination therapy with toripalimab in a patient with meta-oligometastases cervical cancer: a case report., PMID:40260238
Safety and efficacy of neoadjuvant toripalimab plus chemotherapy followed by chemoradiotherapy for locally advanced esophageal squamous cell carcinoma in China (GASTO 1071): a non-randomised, two-cohort, phase 2 trial., PMID:40242562
Evaluation of the efficacy and safety of toripalimab combination therapy for treatment of advanced gastric cancer: a meta-analysis., PMID:40226111
Neoadjuvant toripalimab plus nimotuzumab combined with taxol-based chemotherapy in locally advanced penile squamous cell carcinoma., PMID:40215977
Conversion therapy combined with ALPPS for the treatment of intrahepatic cholangiocarcinoma: a case report., PMID:40190549
[Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma]., PMID:40189973
Cost-effectiveness analysis of toripalimab combined with nab-paclitaxel as a first-line treatment for advanced TNBC in the US., PMID:40168440
[Toripalimab in combination with cisplatin and gemcitabine in recurrent or metastatic nasopharyngeal carcinomas (UCNT RM) in first-line treatment]., PMID:40140319
Efficacy and safety of autologous CIK cell therapy plus Toripalimab with or without chemotherapy in advanced NSCLC: A phase II study., PMID:40135473
Cost-effectiveness of PD-1 inhibitors combined with chemotherapy for first-line treatment of oesophageal squamous cell carcinoma in China: a comprehensive analysis., PMID:40131366
Immune-mediated hepatitis caused by toripalimab: A case report., PMID:40116194
Clinical Manifestations and Risk Factors of Liver Injury Induced by PD-1 Inhibitors in Patients with Malignancies: A Case-Control Study., PMID:40092894
Effect of Early Tumor Shrinkage and Depth of Response on the Clinical Outcomes of Patients with Unresectable Hepatocellular Carcinoma Treated with Transcatheter Arterial Chemoembolization and Lenvatinib plus PD-1 Inhibitors., PMID:40073848
A patient with penile metastasis secondary to small cell lung cancer successfully treated with PD-1 inhibitors and chemotherapy: a case report and literature review., PMID:40071100
Efficacy and safety of targeted therapy and immunotherapy in advanced vulvar squamous cell carcinoma: A scoping review., PMID:40068805
Are all programmed cell death protein 1 inhibitors the same?, PMID:40027132
Long-Term and Sustained Remission of Advanced Triple-Negative Breast Cancer with Large Chest Wall Lesions after Transient Chemoimmunotherapy: A Case Report., PMID:39980521
A phase I dose-escalation and expansion study of RMX1002, a selective E-type prostanoid receptor 4 antagonist, as monotherapy and in combination with anti-PD-1 antibody in advanced solid tumors., PMID:39976872
Toripalimab: A new age in fighting Nasopharyngeal Carcinoma., PMID:39948809
Extended survival of a patient with gastrointestinal multiple malignancies managed with anti-PD-1 immunotherapy: a case report., PMID:39935272
Efficacy and Safety of Low-Dose Lenvatinib and Toripalimab in Patients With Recurrent Platinum-Resistant Ovarian Cancer: Study Protocol of a Multicenter, Open-Label, Single-Arm, Phase II Clinical Trial., PMID:39931671
Long term survival following cryoablation with adjuvant Toripalimab for anorectal malignant melanoma: a case report., PMID:39926280
Anti-LAG-3 antibody LBL-007 plus anti-PD-1 antibody toripalimab in advanced nasopharyngeal carcinoma and other solid tumors: an open-label, multicenter, phase Ib/II trial., PMID:39920751
Efficacy of radiolabelled PD-L1-targeted nanobody in predicting and evaluating the combined immunotherapy and chemotherapy for resectable non-small cell lung cancer., PMID:39912938
Toripalimab plus platinum-doublet chemotherapy as perioperative therapy for initially unresectable NSCLC: An open-label, phase 2 trial., PMID:39892382
Efficacy and safety of surufatinib plus toripalimab in treatment-naive, PD-L1-positive, advanced or metastatic non-small-cell lung cancer and previously treated small-cell lung cancer: an open-label, single-arm, multicenter, multi-cohort phase II trial., PMID:39891720
Comparative efficacy and safety of first-line neoadjuvant therapy for early-stage non-small cell lung cancer based on immune checkpoint inhibitor therapy: a systematic review and network meta-analysis., PMID:39885491
Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy for stage II-IVa esophageal cancer: a network meta-analysis., PMID:39871293