Catalog No.
KDH02206
Description
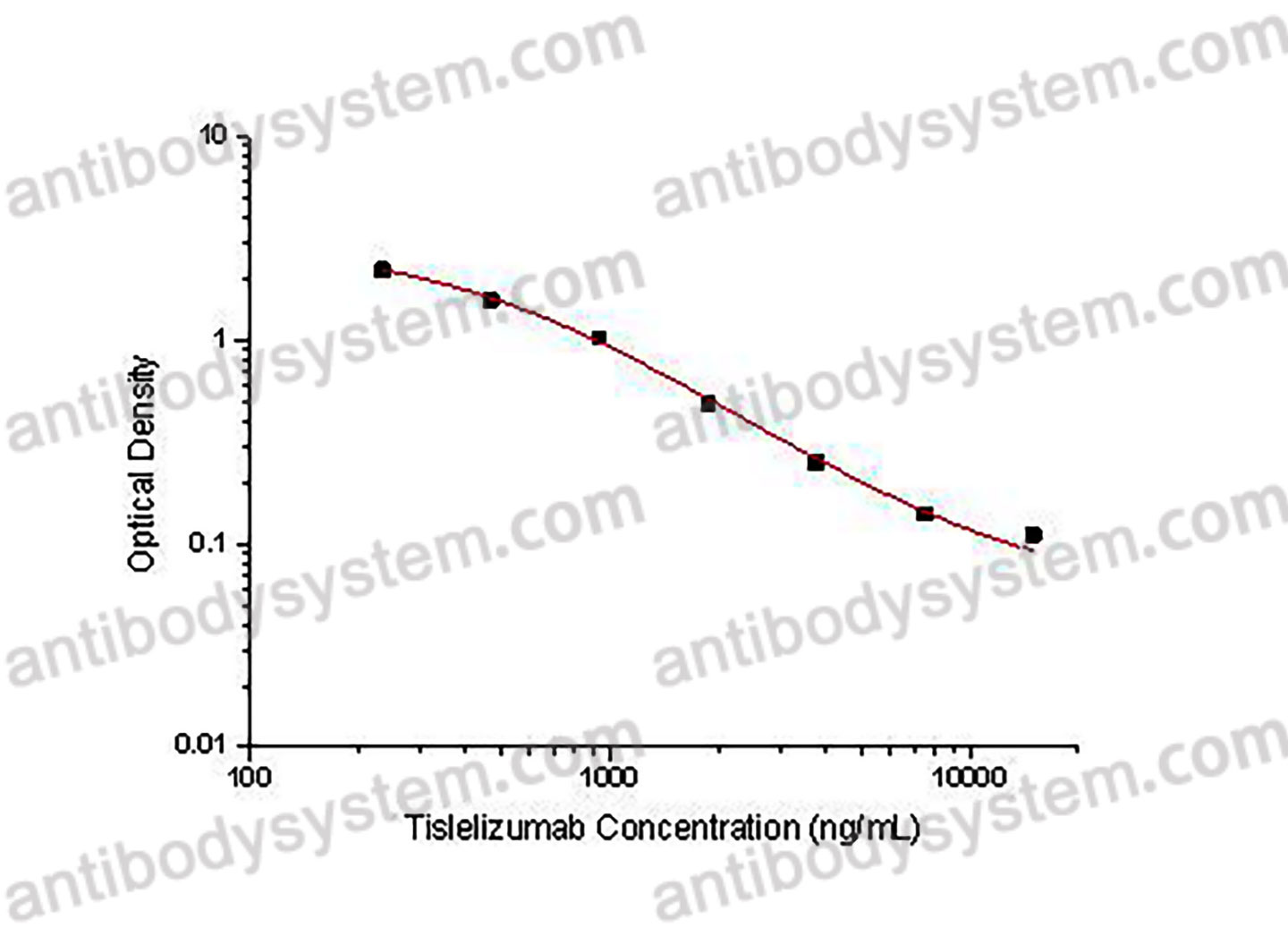
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD279 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tislelizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tislelizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tislelizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Tislelizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
124.74 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
6311.3
|
1419.4
|
370.9
|
5816.8
|
1277.0
|
316.2
|
|
Standard deviation
|
1060.7
|
87.4
|
23.1
|
849.0
|
91.8
|
34.1
|
|
CV (%)
|
16.8
|
6.2
|
6.2
|
14.6
|
7.2
|
10.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BGB-A317, CAS: 1858168-59-8
Case Report: Robust and durable response to the combination of tislelizumab and chemotherapy in advanced thymic epithelial tumors: a case series., PMID:40496871
Efficacy and safety of Tislelizumab combined with Axitinib as first-line treatment for intermediate- and high-risk metastatic clear-cell renal cell carcinoma., PMID:40495894
Post-marketing safety concerns with Tislelizumab: a disproportionality analysis of the FDA adverse event reporting system., PMID:40491908
A rare case of rectal malignant melanoma with long-term survival: case report and literature review., PMID:40483466
Cost-effectiveness analysis of tislelizumab plus chemotherapy as first-line treatment for HER2-negative advanced gastric or gastro-oesophageal junction adenocarcinoma., PMID:40474969
Combined effect of Tislelizumab and chemotherapy on tumor control rate and prognosis in patients with small cell lung cancer., PMID:40469132
Guidelines of Onkopedia: What is new? Esophageal Cancer., PMID:40451170
PD-1 monoclonal antibody (Tislelizumab)-induced DRESS syndrome in an intrahepatic cholangiocarcinoma patient with FGFR3 mutation and elevated IgG4:A case report., PMID:40447060
Primary adenocarcinoma of the renal pelvis: A case report., PMID:40441222
A case of diabetic ketoacidosis triggered by immune checkpoint inhibitors in the treatment of recurrent nasopharyngeal carcinoma., PMID:40439560
Rechallenge of anti-PD-1 antibody combined with chemotherapy shows promising efficacy in the treatment of advanced metastatic hepatocellular carcinoma: A case report., PMID:40438874
Granulomatosis with polyangiitis induced by the anti-programmed cell death-1 inhibitor tislelizumab: A case report., PMID:40420929
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
Efficacy, safety and exploratory analysis of neoadjuvant tislelizumab (a PD-1 inhibitor) plus nab-paclitaxel followed by epirubicin/cyclophosphamide for triple-negative breast cancer: a phase 2 TREND trial., PMID:40414961
Efficacy and safety of combining tislelizumab with capecitabine compared to capecitabine alone in the adjuvant treatment of biliary tract cancers: rationale and protocol design for a randomized clinical trial., PMID:40414848
Tislelizumab as adjuvant therapy following endoscopic surgery for resectable recurrent nasopharyngeal carcinoma: a randomized clinical trial., PMID:40413023
AdvanTIG-202: Phase 2 open-label, two-cohort multicenter study of ociperlimab plus tislelizumab and tislelizumab alone in patients with previously treated recurrent or metastatic cervical cancer., PMID:40411966
[Short-term effects and safety outcomes of the combination of tislelizumab and neoadjuvant chemotherapy in the perioperative treatment of locally advanced gastric cancer]., PMID:40404373
Neoadjuvant arterial infusion chemotherapy combined with immunotherapy in treating locally advanced lower esophageal and esophagogastric junction cancer., PMID:40400978
Psychometric validation of the EORTC QLQ-OES18 in patients with advanced or metastatic esophageal squamous cell carcinoma., PMID:40397297
Insights into Sorafenib resistance in hepatocellular carcinoma: Mechanisms and therapeutic aspects., PMID:40389183
Neoadjuvant with low-dose radiotherapy, tislelizumab, albumin-bound paclitaxel, and cisplatin for resectable locally advanced head and neck squamous cell carcinoma: phase II single-arm trial., PMID:40382318
Complete remission of a high-risk, locally advanced cervical cancer with para-aortic lymph node metastases treated with first-line tislelizumab plus bevacizumab combined with chemotherapy followed by radiotherapy with maintenance therapy: a case report., PMID:40375998
[Analysis of the efficacy of neoadjuvant immunotherapy combined with chemotherapy in non-small cell lung cancer patients after percutaneous coronary intervention]., PMID:40374341
Concordance Between the PD-L1 Tumor Area Positivity Score and Combined Positive Score for Gastric or Esophageal Cancers Treated With Tislelizumab., PMID:40373876
Haematological toxicities with immune checkpoint inhibitors in digestive system tumors: a systematic review and network meta-analysis of randomized controlled trials., PMID:40360867
Clinical manifestations and risk factors of immune-related thyroid adverse events in patients treated with PD-1 inhibitors: a case-control study., PMID:40356893
A case report of mesalazine alleviating diarrhea in a patient with nasopharyngeal cancer after tislelizumab treatment., PMID:40350385
Updates from a single-center phase 2 study of PD-1 inhibitor combined with hypomethylating agent plus CAG regimen in patients with relapsed/refractory acute myeloid leukemia., PMID:40313949
SMARCA4-deficient NSCLC treated with first-line tislelizumab and fruquintinib achieved remarkable tumor regression: case report and literature review., PMID:40313929
Immune checkpoint inhibitor therapy as a neoadjuvant treatment for muscle-invasive bladder carcinoma: A narrative review., PMID:40313419
Unexpected outcomes of tislelizumab treatment in thoracic metastasis of malignant phyllodes tumors: a case report and literature review., PMID:40297816
Clinical Pharmacology Overview of Tislelizumab in Patients With Advanced Tumors With a Focus on Racial Impact., PMID:40286322
Alternative Dosing Regimens of Tislelizumab Using a Pharmacometrics Model-Based Approach., PMID:40286320
Response-adapted zanubrutinib and tislelizumab as a potential strategy to enhance CD19 CAR T-cell therapy in relapsed/refractory large B-cell lymphoma: A retrospective observational study., PMID:40268516
Efficacy and safety of programmed cell death protein-1 inhibitor for first-line therapy of advanced gastric or gastroesophageal junction cancer: a network meta-analysis., PMID:40264761
Tislelizumab for the treatment of advanced esophageal squamous cell carcinoma., PMID:40257370
Pulmonary artery pseudoaneurysm-induced massive hemoptysis after chemotherapy combined with tislelizumab for lung squamous cell carcinoma: a case report., PMID:40248079
Evaluation of changes in price, volume and expenditure of PD-1 drugs following the government reimbursement negotiation in China: a multiple-treatment period interrupted time series analysis., PMID:40247711
Comparative efficacy of tislelizumab plus lenvatinib and tislelizumab alone against advanced hepatocellular carcinoma after lenvatinib failure: a real-world study., PMID:40240993
Efficacy of Tislelizumab in Lung Cancer Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials., PMID:40230743
Polymeric Micellar Paclitaxel Plus Cisplatin Combined With Tislelizumab as the First-Line Treatment of Advanced Unresectable Esophageal Squamous Cell Carcinoma: A Phase II Study., PMID:40228531
Rare Gingival Metastasis Occurring After Conversion Therapy Followed by Resection of Initially Unresectable Hepatocellular Carcinoma: A Case Report., PMID:40226819
Efficacy of presurgical therapy with tislelizumab and axitinib to downsize local lesions in locally advanced and metastatic renal cell carcinoma: a single-institution experience with long-term follow-up., PMID:40226082
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of amivantamab plus lazertinib for non-small cell lung cancer, durvalumab for small cell lung cancer, tislelizumab for esophageal cancer, tisotumab vedotin for cervical cancer, ivosidenib for leukemia, and venetoclax for lymphoma in Japan., PMID:40214878
Impact of tislelizumab combined with albumin-bound paclitaxel and carboplatin on intestinal flora and gastrointestinal toxicities in advanced lung cancer., PMID:40203441
Bevacizumab, tislelizumab and nab-paclitaxel for previously untreated metastatic triple-negative breast cancer: a phase II trial., PMID:40199609
Successful treatment of an elderly patient with lung squamous cell carcinoma by tislelizumab and chemotherapy: a case report with novel imaging findings., PMID:40196110
NOTCH1 Mutation and Survival Analysis of Tislelizumab in Advanced or Metastatic Esophageal Squamous Cell Carcinoma: A Biomarker Analysis From the Randomized, Phase III, RATIONALE-302 Trial., PMID:40179324
Efficacy of combined immunotherapy and targeted therapy in overcoming barriers to postoperative recurrence in squamous subtype anaplastic thyroid carcinoma with abscess: a case report and literature review., PMID:40177243