Catalog No.
KDH02204
Description
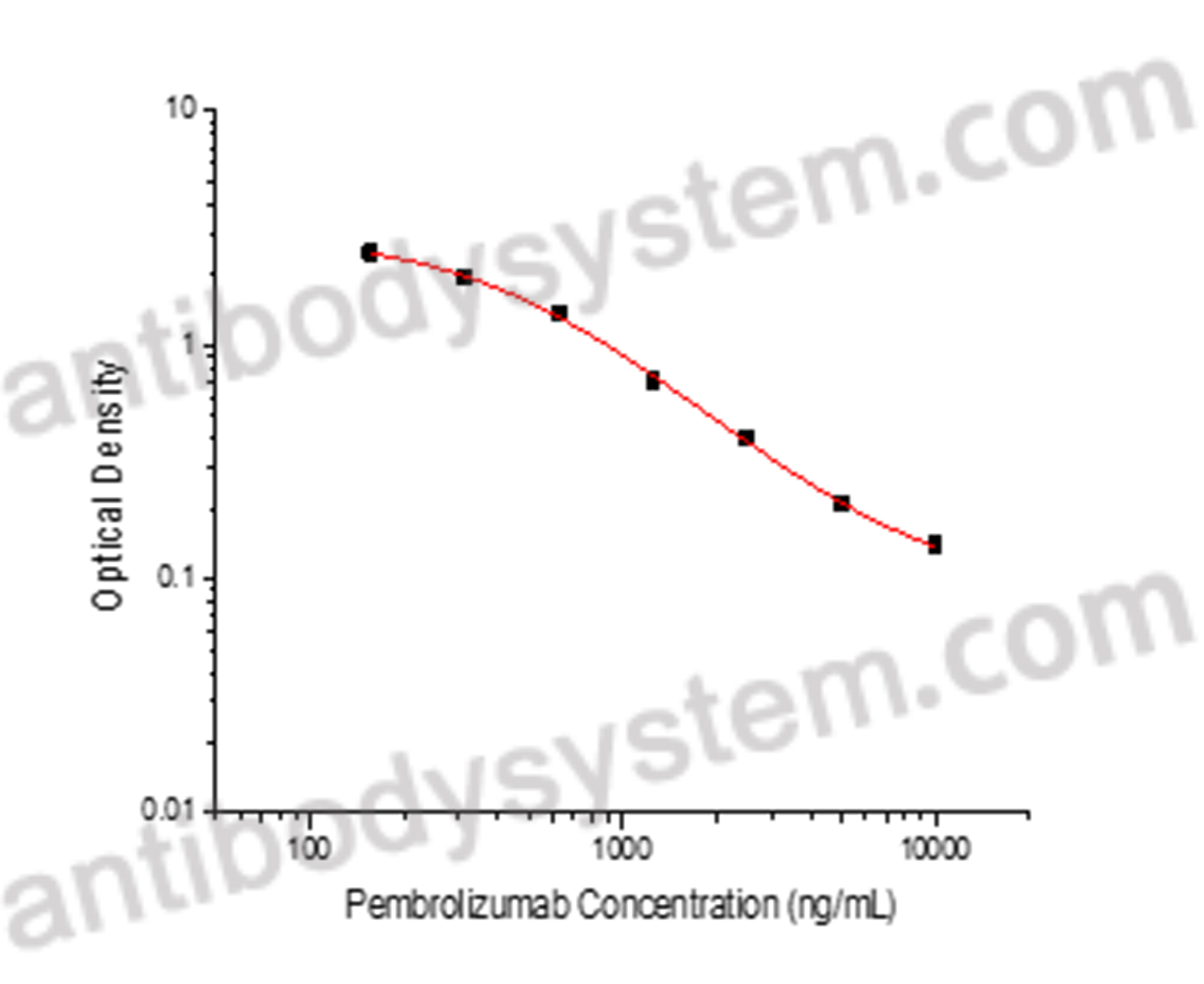
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD279 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Pembrolizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Pembrolizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Pembrolizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Pembrolizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
31.40 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5761.5
|
1291.7
|
334.9
|
5630.5
|
1319.0
|
293.1
|
|
Standard deviation
|
449.5
|
54.3
|
20.3
|
780.4
|
135.3
|
46.1
|
|
CV (%)
|
7.8
|
4.2
|
6.1
|
13.9
|
10.3
|
15.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
MK-3475, CAS: 1374853-91-4
Background
Pembrolizumab is an antibody drug that targets the cell surface receptor programmed cell death protein 1 (PD-1) found on T cells. By preventing the binding of its ligands (PD-L1 and PD-L2), pembrolizumab induces an antitumor immune response. Upregulation of PD-1 ligands is a mechanism for tumors to evade antitumor immune response; when PD-1 binds its ligand, the T cell receives an inhibitory signal leading to T cell anergy and blockade of anti-tumour immune response. Instead of directly targeting tumor tissue to induce tumor cell death, pembrolizumab acts as a checkpoint inhibitor to stimulate immune responses to eliminate cancer cells.
Cardiac outcomes following adjuvant radiotherapy in triple-negative breast cancer patients with prior autoimmune myocarditis., PMID:40527411
Efficacy of pembrolizumab in MSI-high and BRCA-positive castration-resistant prostate cancer., PMID:40527113
Wolf isotopic response: immunotherapy-related lichenoid eruption localizing to prior radiation site., PMID:40526967
Enfortumab vedotin-induced widespread vesiculobullous eruption mimicking disseminated herpetic infection in a patient with metastatic urothelial carcinoma., PMID:40526955
Optimizing enfortumab vedotin plus pembrolizumab therapy., PMID:40526099
Predicting outcomes with pembrolizumab: a meta-analysis of pre-treatment hematological and clinical prognostic factors in advanced/metastatic urothelial carcinoma., PMID:40526042
Immune Checkpoint Inhibitors for Microsatellite Instability High Unresectable Obstructive Colon Cancer: A Report of Two Cases., PMID:40524862
Adjuvant pembrolizumab therapy for completely resected stage I lung adenocarcinoma with micropapillary or solid histological subtypes: a single-center, single-arm, phase 2 trial., PMID:40524802
Aortoesophageal fistula following thoracic branch endoprosthesis for cryptogenic penetrating aortic ulcer in a patient on pembrolizumab., PMID:40521387
Synergistic approach to combating triple-negative breast cancer: DDR1-targeted antibody-drug conjugate combined with pembrolizumab., PMID:40521369
A pilot study investigating the effect of pembrolizumab on the tumoral immunoprofile of newly diagnosed mullerian cancers., PMID:40521348
Immune-Related Fulminant Myocarditis Revealed Using Myocardial Histopathology at Autopsy in the Treatment of Advanced Renal Cell Carcinoma: A Case Report., PMID:40519645
The Efficacy and Safety of Pembrolizumab in Anaplastic Thyroid Carcinoma: A Systematic Review and Meta-Analysis., PMID:40518758
FDG-PET/CT and CT compared for evaluation of tumor response to first-line immunotherapy and prediction of prognosis in non-small-cell lung cancer patients., PMID:40517759
Uncovering prognostic biomarkers through a pharmacovigilance study: the case of RDW., PMID:40517345
Current status of adjuvant immunotherapy and relapse management in renal cell carcinoma: Insights from a European delphi study., PMID:40516320
Avelumab maintenance in advanced urothelial carcinoma: real-world data from Northern Spain (AVEBLADDER study)., PMID:40514610
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Neoadjuvant dose-dense anthracycline and cyclophosphamide in combination with carboplatin, paclitaxel, and pembrolizumab for triple-negative breast cancer: a systematic review and meta-analysis., PMID:40512324
LiGeR-HN phase III trials of petosemtamab + pembrolizumab and petosemtamab monotherapy in recurrent or metastatic HNSCC., PMID:40511820
Pembrolizumab-induced myocarditis, myositis, and myasthenia gravis overlap syndrome (IM3OS) treated with Efgartigimod: case report., PMID:40510022
Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update., PMID:40507360
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy., PMID:40507249
Pembrolizumab plus chemotherapy in advanced endometrial cancer: a cost-effectiveness analysis., PMID:40506696
[Lichen sclerosus induced by pembrolizumab]., PMID:40506272
Management of Cutaneous Melanoma, With a Special Focus on Desmoplastic Melanoma., PMID:40505073
Adjuvant pembrolizumab following kidney cancer surgery A novel patient decision aid to facilitate shared decision-making., PMID:40503866
Managing severe dermatitis and grade 4 pneumonitis in a patient with non-small-cell lung cancer following pembrolizumab treatment: A case report., PMID:40503034
Metastatic renal cell carcinoma to the glans penis: A case report of rare presentation., PMID:40502920
Design and rationale of the phase II PANDORA trial: first line chemo-immunotherapy in advanced Merkel cell carcinoma., PMID:40501309
Phase 1 and 2 Clinical Studies of the STING Agonist Ulevostinag With and Without Pembrolizumab in Participants With Advanced or Metastatic Solid Tumors or Lymphomas., PMID:40499147
Impact of Tumor Response and Response Duration on Survival Among Participants Receiving Pembrolizumab Plus Chemotherapy as First-Line Therapy for Non-Small-Cell Lung Cancer., PMID:40498298
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Enfortumab vedotin induced interstitial lung disease: A first case report with pathological evidence from transbronchial lung cryobiopsy., PMID:40496844
Severe immunotherapy-related thrombocytopenia in metastatic bone cancer: a multicenter retrospective case series highlighting early recognition and management., PMID:40496609
First-line pembrolizumab used concurrently with multi-agent chemotherapy and inhaled tranexamic acid (TXA) for management of stage III mixed trophoblastic tumor complicated by pulmonary hemorrhage., PMID:40496174
Response of EGFR-mutated pulmonary pleomorphic carcinoma to pembrolizumab., PMID:40491684
Pembrolizumab-induced toxic epidermal necrolysis with limited mucosal involvement., PMID:40491518
First-Line Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Retrospective, Multicenter, Real-World Study., PMID:40491285
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Cost-effectiveness of chemotherapy in advanced and recurrent endometrial cancer., PMID:40487854
A Phase 1/1B Trial of Pembrolizumab and Trametinib in Advanced NSCLC Enriched for KRAS Mutations., PMID:40486486
Second-line pembrolizumab for advanced HCC: Meta-analysis of the phase III KEYNOTE-240 and KEYNOTE-394 studies., PMID:40486134
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of belantamab mafodotin for multiple myeloma, asciminib for leukemia, osimertinib for lung cancer, amivantamab for lung cancer, and pembrolizumab for pleural mesothelioma in Japan., PMID:40481943
Two Cases of Enfortumab Vedotin-Induced Drug Eruption Diagnosed Based on the Distinctive Epidermal Mitotic Figures in Patients Receiving Combination Therapy With Pembrolizumab., PMID:40476598
Immune checkpoint inhibitor therapy in metastatic renal cell carcinoma: tumour response and immune-related renal vasculitis following cytoreductive nephrectomy., PMID:40474803