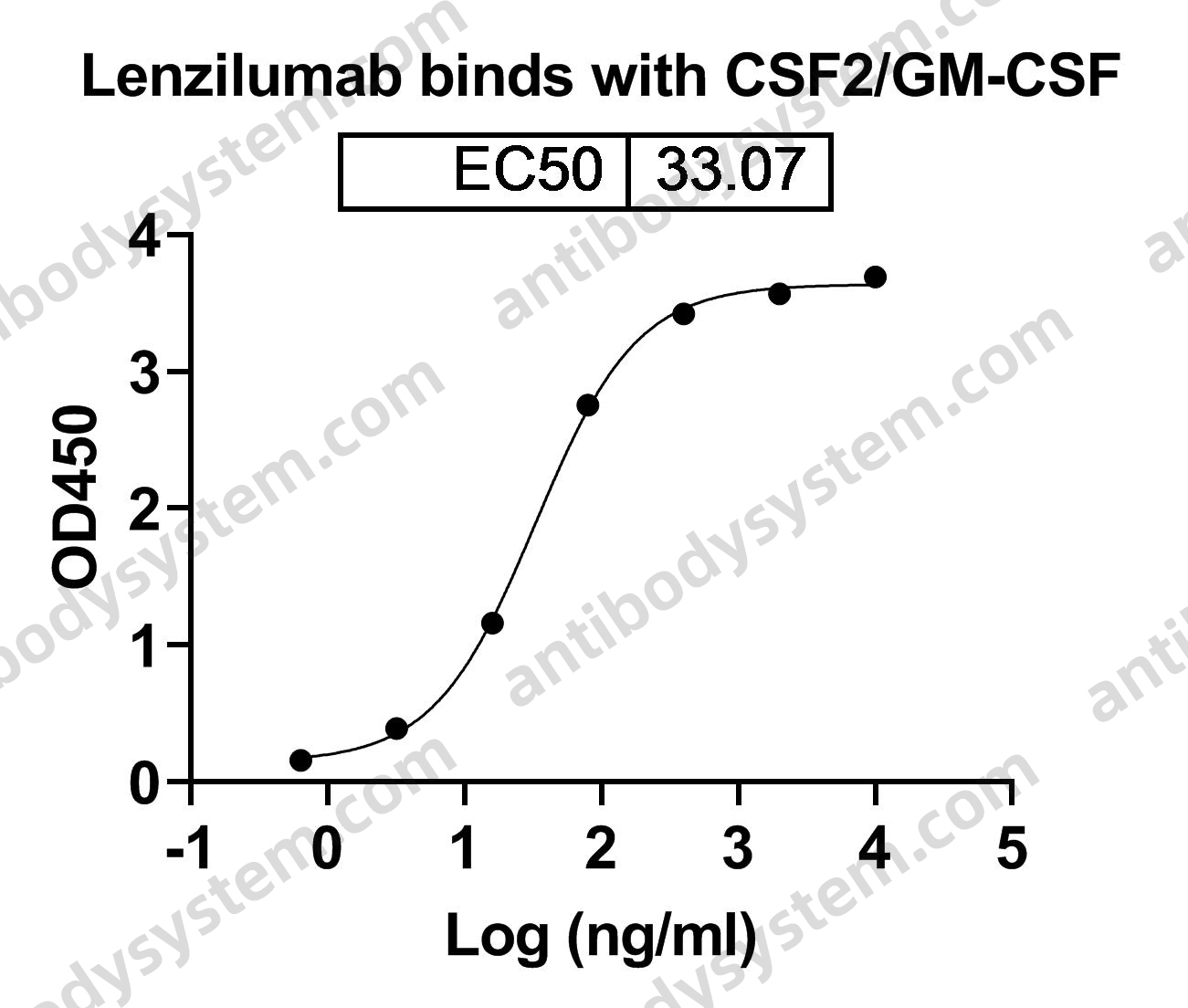
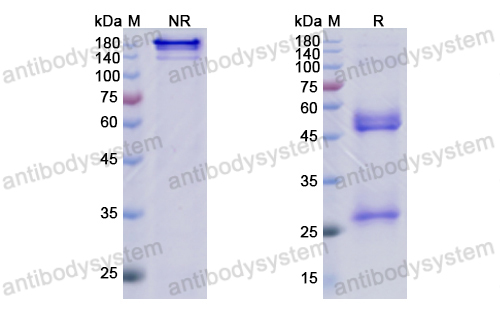
Catalog No.
DHC06703
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Granulocyte-macrophage colony-stimulating factor, Sargramostim, GMCSF, CSF, Colony-stimulating factor, CSF2, GM-CSF, Molgramostin
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P04141
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
KB-003, CAS: 1229575-09-0
Clone ID
Lenzilumab
Case report: use of lenzilumab and tocilizumab for the treatment of coronavirus disease 2019, PMID: 32546029
Clinical Benefit of Lenzilumab in Cases of Coronavirus Disease 2019, PMID: 33673929
Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 Pneumonia Who Developed Bradycardia, PMID: 34215897
First Clinical Use of Lenzilumab to Neutralize GM-CSF in Patients with Severe COVID-19 Pneumonia, PMID: 32587983
GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts, PMID: 30463995
GM-CSF Neutralization With Lenzilumab in Severe COVID-19 Pneumonia: A Case-Cohort Study, PMID: 33153629
In Reply - Clinical Benefit of Lenzilumab in Cases of Coronavirus Disease 2019, PMID: 33673930
Increasing recognition and emerging therapies argue for dedicated clinical trials in chronic myelomonocytic leukemia, PMID: 34175902
LENZILUMAB EFFICACY AND SAFETY IN NEWLY HOSPITALIZED COVID-19 SUBJECTS: RESULTS FROM THE LIVE-AIR PHASE 3 RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED TRIAL, PMID: 33972949
Pharmaco-Immunomodulatory Therapy in COVID-19, PMID: 32696108
Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia, PMID: 32294158
Risk-Adapted, Individualized Treatment Strategies of Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML), PMID: 33807279
Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies, PMID: 32719685
Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof), PMID: 32561148
A Glimpse for the subsistence from pandemic SARS-CoV-2 infection., PMID:39603070
Reduction of therapeutic antibody self-association using yeast-display selections and machine learning., PMID:36433737
[Current and future therapeutic options for COVID-19]., PMID:36036702
C reactive protein utilisation, a biomarker for early COVID-19 treatment, improves lenzilumab efficacy: results from the randomised phase 3 'LIVE-AIR' trial., PMID:35793833
Clinical and Economic Benefits of Lenzilumab Plus Standard of Care Compared with Standard of Care Alone for the Treatment of Hospitalized Patients with Coronavirus Disease 19 (COVID-19) from the Perspective of National Health Service England., PMID:35444434
In inpatients with COVID-19 who need supplemental oxygen, lenzilumab increased ventilation-free survival., PMID:35377717
Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review., PMID:35126138
Clinical and economic benefits of lenzilumab plus standard of care compared with standard of care alone for the treatment of hospitalized patients with COVID-19 in the United States from the hospital perspective., PMID:35037816
Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial., PMID:34863332
Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 Pneumonia Who Developed Bradycardia., PMID:34215897
Increasing recognition and emerging therapies argue for dedicated clinical trials in chronic myelomonocytic leukemia., PMID:34175902
LENZILUMAB EFFICACY AND SAFETY IN NEWLY HOSPITALIZED COVID-19 SUBJECTS: RESULTS FROM THE LIVE-AIR PHASE 3 RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED TRIAL., PMID:33972949
Risk-Adapted, Individualized Treatment Strategies of Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML)., PMID:33807279
In Reply - Clinical Benefit of Lenzilumab in Cases of Coronavirus Disease 2019., PMID:33673930
Clinical Benefit of Lenzilumab in Cases of Coronavirus Disease 2019., PMID:33673929
GM-CSF Neutralization With Lenzilumab in Severe COVID-19 Pneumonia: A Case-Cohort Study., PMID:33153629
Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies., PMID:32719685
Pharmaco-Immunomodulatory Therapy in COVID-19., PMID:32696108
First Clinical Use of Lenzilumab to Neutralize GM-CSF in Patients with Severe COVID-19 Pneumonia., PMID:32587983
Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof)., PMID:32561148
Case report: use of lenzilumab and tocilizumab for the treatment of coronavirus disease 2019., PMID:32546029
Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia., PMID:32294158
GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts., PMID:30463995