Catalog No.
KDG52002
Description
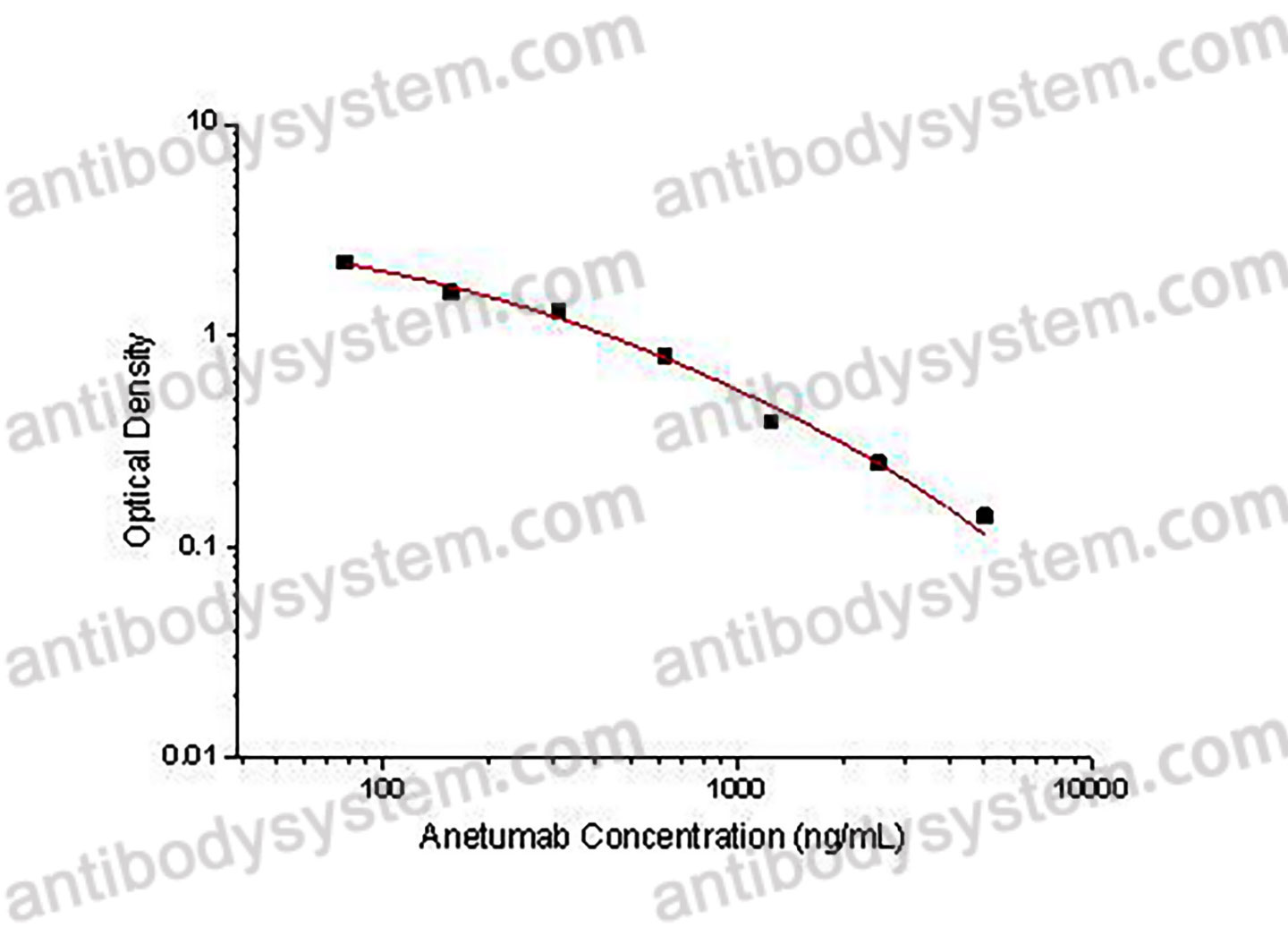
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human MSLN has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Anetumab in the sample competitively binds to the pre-coated protein with biotin-labeled Anetumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anetumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anetumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
78.13 - 5,000 ng/mL
Sensitivity
55.57 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1785.1
|
538.9
|
145.1
|
2013.7
|
451.7
|
120.9
|
|
Standard deviation
|
163.0
|
62.0
|
28.0
|
221.2
|
63.1
|
18.7
|
|
CV (%)
|
9.1
|
11.5
|
19.3
|
11.0
|
14.0
|
15.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BAY 94-9343, BAY 86-1903, BAY 2287409, Anetumab Ravtansine, CAS: 1954758-84-9
Background
Anetumab ravtansine, also known as BAY 94-9343, is a novel, selective and highly potent antibody-drug conjugate consisting of a human anti-mesothelin antibody conjugated to the maytansinoid tubulin inhibitor DM4 via a disulfide-containing linker (a reducible SPDB linker [N-succinimidyl 4- (2-pyridyldithio)butanoate]) on an average of 3 lysyl.
Evaluating therapeutic plasma exchange and protease inhibitors as mechanisms to reduce soluble mesothelin., PMID:40240561
Evaluation of Innate Immune System, Body Habitus, and Sex on the Pharmacokinetics and Pharmacodynamics of Anetumab Ravtansine in Patients With Cancer., PMID:40051118
Development of a novel molecular probe for visualizing mesothelin on the tumor via positron emission tomography., PMID:39878895
Randomized Phase II Study of Bevacizumab with Weekly Anetumab Ravtansine or Weekly Paclitaxel in Platinum-Resistant/Refractory High-Grade Ovarian Cancer (NCI Trial)., PMID:39836408
Randomized trial of anetumab ravtansine and pembrolizumab compared to pembrolizumab for mesothelioma., PMID:39197359
Mesothelin promotes brain metastasis of non-small cell lung cancer by activating MET., PMID:38570866
Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study., PMID:36564099
Anetumab ravtansine versus vinorelbine in patients with relapsed, mesothelin-positive malignant pleural mesothelioma (ARCS-M): a randomised, open-label phase 2 trial., PMID:35358455
Salvage Therapy for Relapsed Malignant Pleural Mesothelioma: A Systematic Review and Network Meta-Analysis., PMID:35008346
Exploiting mesothelin in thymic carcinoma as a drug delivery target for anetumab ravtansine., PMID:34876673
In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab., PMID:34014767
Mesothelin is a novel cell surface disease marker and potential therapeutic target in acute myeloid leukemia., PMID:33938941
Novel mesothelin antibody-drug conjugates: current evidence and future role in the treatment of ovarian cancer., PMID:33356644
Favorable therapeutic response after anti-Mesothelin antibody-drug conjugate treatment requires high expression of Mesothelin in tumor cells., PMID:32815024
Mesothelin expression in esophageal adenocarcinoma and squamous cell carcinoma and its possible impact on future treatment strategies., PMID:32547645
First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors., PMID:32213105
Antibody-drug conjugates in gynecologic malignancies., PMID:30929824
Targeting mesothelin in ovarian cancer., PMID:30546824
Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models., PMID:30344925
Mesothelin as a target for cervical cancer therapy., PMID:30324544
[Systemic Treatment of Malignant Pleural Mesothelioma]., PMID:29361614
Antibodies to watch in 2017., PMID:27960628
Mesothelin Immunotherapy for Cancer: Ready for Prime Time?, PMID:27863199
Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors., PMID:27853672
Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect., PMID:24714131