Catalog No.
KDG17601
Description
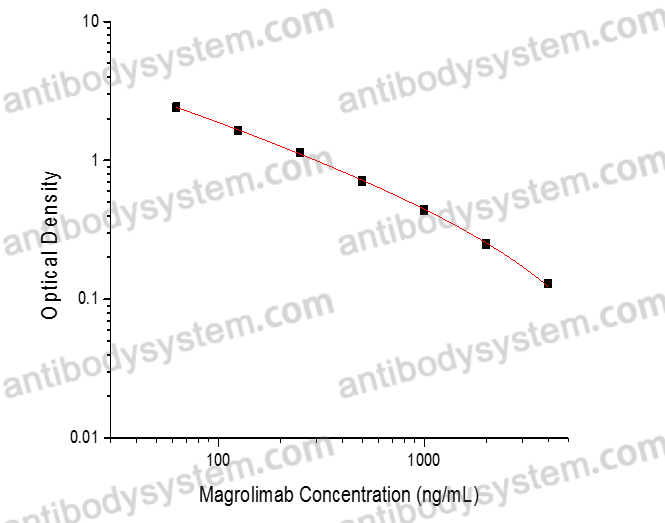
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD47 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Magrolimab in the sample competitively binds to the pre-coated protein with biotin-labeled Magrolimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Magrolimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Magrolimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
48.26 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2786.9
|
629.3
|
146.2
|
2300.7
|
527.9
|
124.3
|
|
Standard deviation
|
206.5
|
95.9
|
10.1
|
353.8
|
62.4
|
23.6
|
|
CV (%)
|
7.4
|
15.2
|
6.9
|
15.4
|
11.8
|
19.0
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
Hu5F9-G4, CAS: 2169232-81-7
Background
Magrolimab is a first-in-class investigational monoclonal antibody against CD47 and macrophage checkpoint inhibitor that is designed to interfere with recognition of CD47 by the SIRPα receptor on macrophages, thus blocking the
Final Results of a Phase 2 Multi-Arm Study of Magrolimab Combinations in Patients With Relapsed/Refractory Multiple Myeloma., PMID:40485906
Phase 2 Multi-Arm Study of Magrolimab Combinations in Patients With Acute Myeloid Leukaemia., PMID:40364806
Magrolimab Therapy in Conjunction with Conventional Chemotherapeutics Slows Disease Progression in Pediatric Acute Myeloid Leukemia Patient-Derived Xenograft Models., PMID:40361435
The ENHANCE-3 study: venetoclax and azacitidine plus magrolimab or placebo for untreated AML unfit for intensive therapy., PMID:40233321
Azacitidine, Venetoclax, and Magrolimab in Newly Diagnosed and Relapsed Refractory Acute Myeloid Leukemia: Phase Ib/II Study and Correlative Analysis., PMID:40198272
A Phase 1b/2 Study of the Anti-CD47 Antibody Magrolimab with Cetuximab in Patients with Colorectal Cancer and Other Solid Tumors., PMID:40140179
Magrolimab plus azacitidine vs physician's choice for untreated TP53-mutated acute myeloid leukemia: the ENHANCE-2 study., PMID:40009500
Macrophages as Potential Therapeutic Targets in Acute Myeloid Leukemia., PMID:39457618
Managing the unmanageable: evidence-driven approaches to real-world patient prototypes of TP53-mutant myelodysplastic neoplasms and acute myeloid leukemia., PMID:39349613
Magrolimab plus rituximab with or without chemotherapy in patients with relapsed/refractory diffuse large B-cell lymphoma., PMID:39293083
Circumventing resistance within the Ewing sarcoma microenvironment by combinatorial innate immunotherapy., PMID:39266215
Magrolimab plus rituximab in relapsed/refractory indolent non-Hodgkin lymphoma: 3-year follow-up of a phase 1/2 trial., PMID:39213421
Targeting the CD47/SIRPα pathway in malignancies: recent progress, difficulties and future perspectives., PMID:39040441
Combinatorial macrophage induced innate immunotherapy against Ewing sarcoma: Turning "Two Keys" simultaneously., PMID:38992659
Comparison of dose selection based on target engagement versus inhibition of receptor-ligand interaction for checkpoint inhibitors., PMID:38790133
TP53 Mutations in Acute Leukemias and Myelodysplastic Syndromes: Insights and Treatment Updates., PMID:38768424
How do transfusion services manage patients taking therapies such as anti-CD38 and anti-CD47 known to interfere with red blood cell compatibility testing?, PMID:38767410
Is drug interference still an issue for pretransfusion testing of patients on anti CD38 and other monoclonal antibody therapies?, PMID:38705581
Opportunities and challenges for anti-CD47 antibodies in hematological malignancies., PMID:38464520
Treatment outcomes of venetoclax-combination regimens for relapsed/refractory myeloid malignancies after anti-CD47-directed therapy., PMID:38441062
Transient red blood cell agglutination after Magrolimab administration in acute myeloid leukemia., PMID:38366740
Novel immunotherapies in the treatment of AML: is there hope?, PMID:38066884
Frontline treatment options for higher-risk MDS: can we move past azacitidine?, PMID:38066872
Transfusion management in the era of magrolimab (Hu5F9-G4), an anti-CD47 monoclonal antibody therapy., PMID:37970740
A humanized orthotopic mouse model for preclinical evaluation of immunotherapy in Ewing sarcoma., PMID:37868989
TP53-mutated acute myeloid leukemia and myelodysplastic syndrome: biology, treatment challenges, and upcoming approaches., PMID:37770618
Harnessing autologous immune effector mechanisms in acute myeloid leukemia: 2023 update of trials and tribulations., PMID:37729719
Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined With Azacitidine in Patients With Previously Untreated AML: Phase Ib Results., PMID:37703506
Atezolizumab plus Magrolimab, Niraparib, or Tocilizumab versus Atezolizumab Monotherapy in Platinum-Refractory Metastatic Urothelial Carcinoma: A Phase Ib/II Open-Label, Multicenter, Randomized Umbrella Study (MORPHEUS Urothelial Carcinoma)., PMID:37651261
Building on Foundations: Venetoclax-Based Combinations in the Treatment of Acute Myeloid Leukemia., PMID:37509251
Acute Myeloid Leukemia Treatment in the Elderly: A Comprehensive Review of the Present and Future., PMID:37459852
Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study., PMID:36888930
[Management of AML in the elderly]., PMID:36870810
Novel agents and regimens in acute myeloid leukemia: latest updates from 2022 ASH Annual Meeting., PMID:36869366
A phase II multi-arm study of magrolimab combinations in patients with relapsed/refractory multiple myeloma., PMID:36779512
The clinical impact of the molecular landscape of acute myeloid leukemia., PMID:36722402
An overview of novel therapies in advanced clinical testing for acute myeloid leukemia., PMID:36718500
Biological therapy in elderly patients with acute myeloid leukemia., PMID:36715330
Advances in myelodysplastic syndromes: promising novel agents and combination strategies., PMID:36620919
Dual checkpoint blockade of CD47 and LILRB1 enhances CD20 antibody-dependent phagocytosis of lymphoma cells by macrophages., PMID:36389667
Molecular targets for the treatment of AML in the forthcoming 5th World Health Organization Classification of Haematolymphoid Tumours., PMID:36271671
Updates on the Management of Acute Myeloid Leukemia., PMID:36230677
Current status of phase 3 clinical trials in high-risk myelodysplastic syndromes: pitfalls and recommendations., PMID:36215988
How I Treat TP53-Mutated Acute Myeloid Leukemia and Myelodysplastic Syndromes., PMID:36139679
Guidance for transfusion management in patients receiving magrolimab therapy (anti-CD47 monoclonal antibody)., PMID:36114660
Recent Advances in the Therapeutic Armamentarium of Acute Myeloid Leukemia: A Focus on the 63rd American Society of Hematology Annual Meeting Abstracts., PMID:36093823
Myeloid checkpoint blockade improves killing of T-acute lymphoblastic leukemia cells by an IgA2 variant of daratumumab., PMID:36052078
Follicular lymphoma: The long and winding road leading to your cure?, PMID:35908982
New Frontiers in Monoclonal Antibodies for the Targeted Therapy of Acute Myeloid Leukemia and Myelodysplastic Syndromes., PMID:35886899
Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes., PMID:35883692