Catalog No.
KDG11001
Description
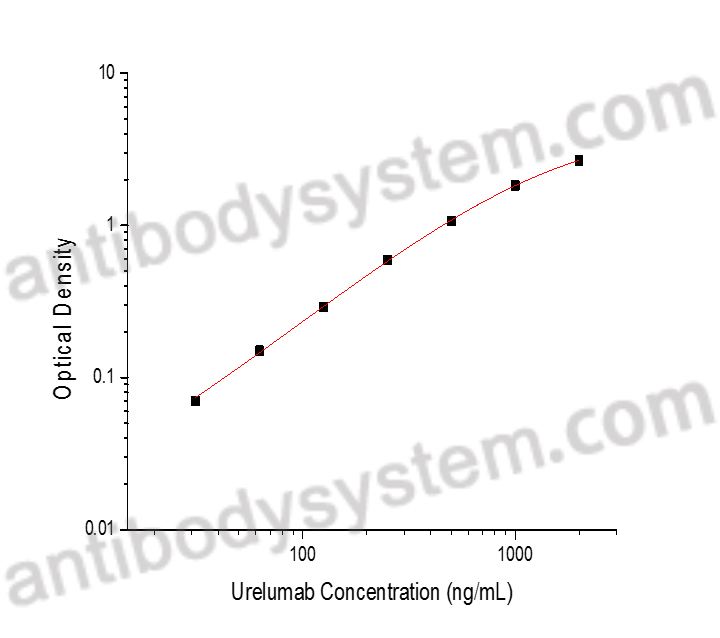
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human CD137 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Urelumab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Urelumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Urelumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
18.73 ng/mL
Precision
PRECISION
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1043.6
|
246.7
|
55.1
|
1054.4
|
255.2
|
71.6
|
|
Standard deviation
|
52.5
|
15.7
|
2.2
|
45.4
|
11.8
|
8.2
|
|
CV (%)
|
5.0
|
6.4
|
4.0
|
4.3
|
4.6
|
11.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
BMS-663513, CAS: 934823-49-1
Background
Urelumab is a fully humanized agonistic monoclonal antibody targeting the CD137 receptor with potential immunostimulatory and antineoplastic activities. Urelumab specifically binds to and activates CD137-expressing immune cells, stimulating an immune response, in particular a cytotoxic T cell response, against tumor cells. Urelumab has been used in trials studying the treatment of Leukemia, Multiple Myeloma, Malignant Tumors, and Cancer-Solid Tumors and B-Cell Non-Hodgkin's Lymphoma. The antibody product was developed using Medarex's UltiMAb(R) technology and was the first UltiMAb-derived antibody in clinical development by Bristol-Myers Squibb under the December 2003 agreement with Medarex. In December 2008, enrolment was stopped for all urelumab studies following the occurrence of two hepatotoxicity-related deaths. Several years later, the clinical study on Urelumab was restarted. At present, the approval of the monoclonal antibody has not been disclosed in the relevant drug approval agency.
Rationale and feasibility of a rapid integral biomarker program that informs immune-oncology clinical trials: the ADVISE trial., PMID:40389374
Development of a tumor-region-selective activation monoclonal antibody targeting the 4-1BB receptor for enhanced therapeutic efficacy and safety., PMID:39978521
Safety of combined ablative radiotherapy and immune checkpoint inhibitors in three phase I trials., PMID:39106643
CB307: A Dual Targeting Costimulatory Humabody VH Therapeutic for Treating PSMA-Positive Tumors., PMID:38593226
Development of a c-MET x CD137 bispecific antibody for targeted immune agonism in cancer immunotherapy., PMID:38492435
Final results of urelumab, an anti-CD137 agonist monoclonal antibody, in combination with cetuximab or nivolumab in patients with advanced solid tumors., PMID:38458639
Efficacy and safety of autologous tumor-infiltrating lymphocytes in recurrent or refractory ovarian cancer, colorectal cancer, and pancreatic ductal adenocarcinoma., PMID:38309721
M9657 Is a Bispecific Tumor-Targeted Anti-CD137 Agonist That Induces MSLN-Dependent Antitumor Immunity without Liver Inflammation., PMID:38091375
Costimulatory capacity of CD137 mAbs on T cells depends on elaborate CRD structures but not on blocking ligand-receptor binding., PMID:37675596
FcγR requirements and costimulatory capacity of Urelumab, Utomilumab, and Varlilumab., PMID:37575254
4-1BB Targeting Immunotherapy: Mechanism, Antibodies, and Chimeric Antigen Receptor T., PMID:37433196
A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma., PMID:37339979
Engineered soluble, trimerized 4-1BBL variants as potent immunomodulatory agents., PMID:37310433
The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy., PMID:36727218
CD137 (4-1BB)-Based Cancer Immunotherapy on Its 25th Anniversary., PMID:36576322
Modulation of urelumab glycosylation separates immune stimulatory activity from organ toxicity., PMID:36248847
Development and characterization of a novel human CD137 agonistic antibody with anti-tumor activity and a good safety profile in non-human primates., PMID:36176235
A humanized 4-1BB-targeting agonistic antibody exerts potent antitumor activity in colorectal cancer without systemic toxicity., PMID:36076251
In vivo validation of the switch antibody concept: SPECT/CT imaging of the anti-CD137 switch antibody Sta-MB shows high uptake in tumors but low uptake in normal organs in human CD137 knock-in mice., PMID:35820360
Soluble CD137 as a dynamic biomarker to monitor agonist CD137 immunotherapies., PMID:35236742
Combined IL-2, agonistic CD3 and 4-1BB stimulation preserve clonotype hierarchy in propagated non-small cell lung cancer tumor-infiltrating lymphocytes., PMID:35110355
CD137 Costimulation Counteracts TGFβ Inhibition of NK-cell Antitumor Function., PMID:34580116
Antitumor efficacy and reduced toxicity using an anti-CD137 Probody therapeutic., PMID:34172583
Phase I Study of Stereotactic Body Radiotherapy plus Nivolumab and Urelumab or Cabiralizumab in Advanced Solid Tumors., PMID:34168049
CD137 as an Attractive T Cell Co-Stimulatory Target in the TNFRSF for Immuno-Oncology Drug Development., PMID:34064598
4-1BB costimulation promotes bystander activation of human CD8 T cells., PMID:33180337
Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma., PMID:32052473
Epitope and Fc-Mediated Cross-linking, but Not High Affinity, Are Critical for Antitumor Activity of CD137 Agonist Antibody with Reduced Liver Toxicity., PMID:31974274
Daratumumab in combination with urelumab to potentiate anti-myeloma activity in lymphocyte-deficient mice reconstituted with human NK cells., PMID:31143521
Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity., PMID:31105267
Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab., PMID:30410017
CD137 (4-1BB) Costimulation Modifies DNA Methylation in CD8+ T Cell-Relevant Genes., PMID:29133290
Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies., PMID:29118009
Checkpoint Inhibition in Non-Hodgkin's Lymphoma., PMID:29065421
4-1BB-Enhanced Expansion of CD8+ TIL from Triple-Negative Breast Cancer Unveils Mutation-Specific CD8+ T Cells., PMID:28473315
Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody., PMID:27756788
CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer., PMID:27496866
Functional expression of CD137 (4-1BB) on T helper follicular cells., PMID:26587331
Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications., PMID:26137403
Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2-/-IL2Rγnull Immunodeficient Mice., PMID:26113085
Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types., PMID:23460532
The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment., PMID:23452415