Catalog No.
KDF92401
Description
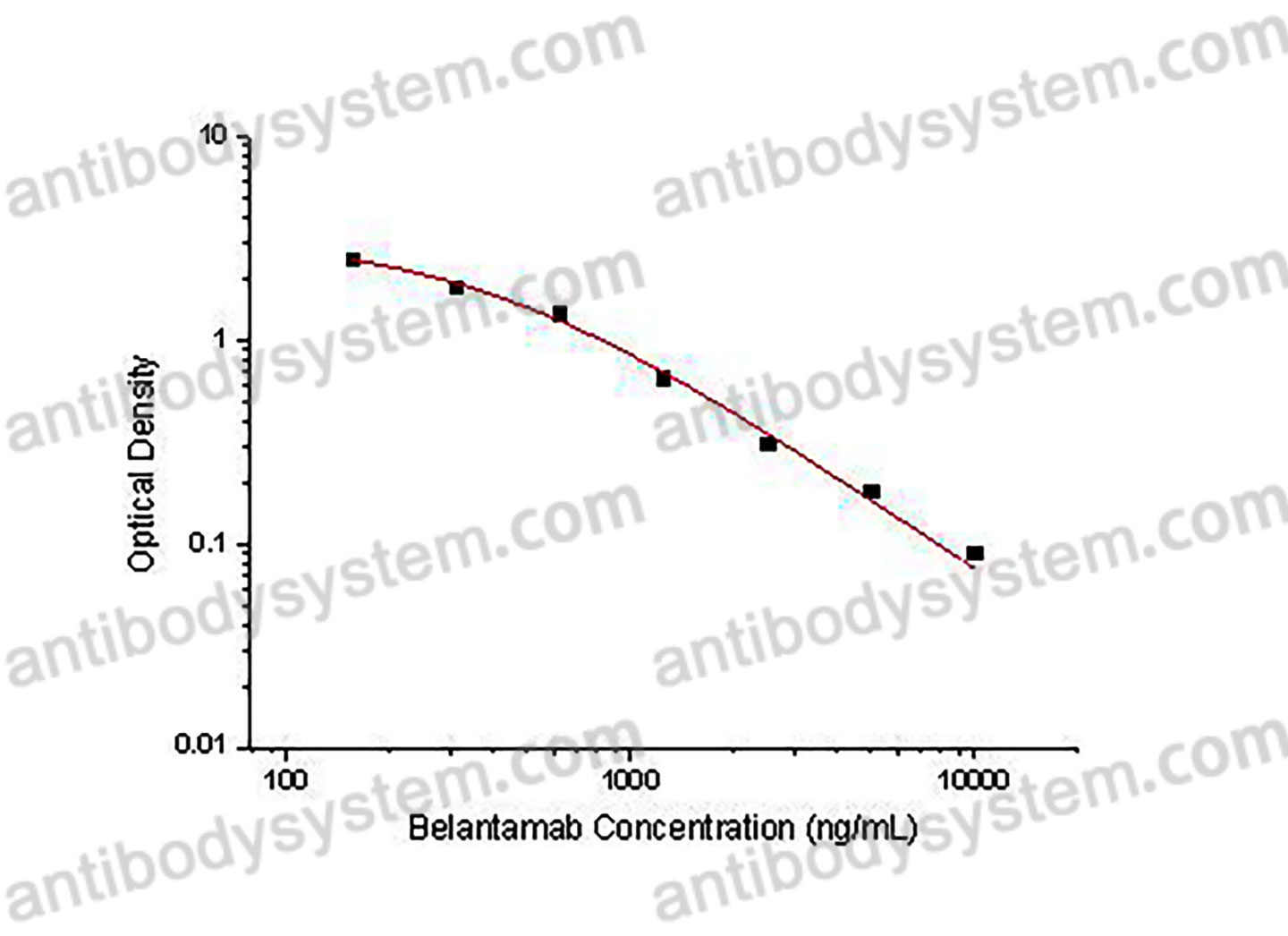
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD269 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Belantamab in the sample competitively binds to the pre-coated protein with biotin-labeled Belantamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Belantamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Belantamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
102.61 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
3827.5
|
1054.7
|
269.2
|
3816.4
|
1049.4
|
266.4
|
|
Standard deviation
|
193.6
|
112.4
|
22.0
|
214.3
|
136.3
|
27.4
|
|
CV (%)
|
5.1
|
10.7
|
8.2
|
5.6
|
13.0
|
10.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
2857916, GSK2857916, J6M0, J6M0-mcMMAF, CAS: 2061894-48-0
Background
Belantamab mafodotin also known as GSK2857916 is a humanized, IgG1 antibody-drug conjugate (ADC) that binds specifically to BCMA. The parent anti-BCMA antibody (GSK2857914) is produced in an afucosylated form and is conjugated to the microtubule polymerization inhibitor MMAF, via a protease-resistant maleimidocaproyl (mc) linker, to produce the GSK2857916 ADC molecule. Upon binding to the cell surface, GSK2857916 is rapidly internalized and active cytotoxic drug (cys-mcMMAF) is released inside the cell causing cell-cycle arrest and apoptosis. Furthermore, the afucosylation of GSK2857916 leads to enhanced binding to FcγRIIIa receptors on the surface of immune effector cells, recruitment of immune cells, and antibody-dependent cellular cytotoxicity and phagocytosis (ADCC and ADCP, respectively). This dual mechanism of action enables improved efficacy by targeting both dividing (via ADC) and non-dividing (via ADCC/ADCP) tumor cells. Recently, belantamab mafodotin was approved under the name BLENREP in the United States and European Union for the treatment of patients with relapsed or refractory multiple myeloma.
An Anti-BCMA Affibody Affinity Protein for Therapeutic and Diagnostic Use in Multiple Myeloma., PMID:40507995
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of belantamab mafodotin for multiple myeloma, asciminib for leukemia, osimertinib for lung cancer, amivantamab for lung cancer, and pembrolizumab for pleural mesothelioma in Japan., PMID:40481943
Real-world characteristics and outcomes of patients with multiple myeloma treated with belantamab mafodotin: a German claims data study., PMID:40448822
Population Pharmacokinetics for Belantamab Mafodotin Monotherapy and Combination Therapies in Patients with Relapsed/Refractory Multiple Myeloma., PMID:40447948
Practice-changing updates on multiple myeloma: highlights from the 2024 ASH annual meeting., PMID:40361170
Treatment Patterns, Efficacy, and Tolerability of Belantamab Mafodotin in Patients With Relapsed and/or Refractory Multiple Myeloma: A Real-World Analysis., PMID:40348718
Characterization of Belantamab Mafodotin-Induced Corneal Changes in Patients With Multiple Myeloma., PMID:40338596
Corneal Findings in Patients Treated with Belantamab Mafodotin: A Prospective Case Series Focusing on Corneal Nerves., PMID:40327294
Belantamab Mafodotin Monotherapy for Multiply-Relapsed Myeloma: A Retrospective Study From the United Kingdom and the Republic of Ireland., PMID:40308262
HSR25-172: Meta-Analysis of Phase 3 Randomized Controlled Trials to Evaluate the Incidence of Infectious Adverse Events in Patients With Relapsed/Refractory Multiple Myeloma Treated With Belantamab Mafodotin., PMID:40154473
HSR25-143: Meta-Analysis of Phase 3 Randomized Controlled Trials to Evaluate the Incidence of Systemic Toxicities in Patients With Relapsed/Refractory Multiple Myeloma Treated With Belantamab Mafodotin., PMID:40154424
Belantamab Mafodotin Plus Proteasome Inhibition Efficacy Versus Comparators in Early Relapsed Myeloma: A Systematic Review and Network Meta-Analysis., PMID:40143674
Correction: The clinical journey of belantamab mafodotin in relapsed or refractory multiple myeloma: lessons in drug development., PMID:40140348
Current Treatment Strategies for Multiple Myeloma at First Relapse., PMID:40095642
Unveiling cardiovascular and respiratory toxicities with monoclonal antibodies in multiple myeloma: disproportionality analysis from the FDA Adverse Event Reporting System., PMID:40095047
Novelties on Multiple Myeloma from the Main 2024 Hematology Conferences., PMID:40084104
A real-world experience of efficacy and safety of belantamab mafodotin in relapsed refractory multiple myeloma., PMID:40064854
Prior exposure to belantamab mafodotin influences outcomes with idecabtagene vicleucel in patients with multiple myeloma., PMID:39938011
The clinical journey of belantamab mafodotin in relapsed or refractory multiple myeloma: lessons in drug development., PMID:39920159
Novel Treatment Options for Multiple Myeloma., PMID:39772633
Update on B-cell maturation antigen-directed therapies in AL amyloidosis., PMID:39748220
DREAMM-11, Part 2: Japanese phase I trial of belantamab mafodotin combination therapies in relapsed/refractory multiple myeloma., PMID:39718747
The Antibody Drug Conjugate, Belantamab-Mafodotin, in the Treatment of Multiple Myeloma: A Comprehensive Review., PMID:39664714
Efficacy and Safety of Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell for the Treatment of Relapsed and Refractory AL Amyloidosis., PMID:39653116
Novel Drug Combinations and Donor Lymphocyte Infusions Allow Prolonged Disease Control in Multiple Myeloma Patients Relapsing after Allogeneic Transplantation., PMID:39505212
Focal and Segmental Glomerulosclerosis in a Multiple Myeloma Patient After Belantamab Mafodotin Therapy and Severe COVID-19 Infection: A Case Report., PMID:39482831
Neuropsychiatric Adverse Events with Monoclonal Antibodies Approved for Multiple Myeloma: An Analysis from the FDA Adverse Event Reporting System., PMID:39458907
Strengths and Weaknesses of Different Therapeutic Strategies for the Treatment of Patients with Multiple Myeloma Who Progress After the Frontline Use of Lenalidomide: A Narrative Review., PMID:39458188
Results from Arm A of Phase 1/2 DREAMM-6 trial: belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma., PMID:39433730
Belantamab mafodotin monotherapy for relapsed or refractory multiple myeloma: a real-world observational study in the United States., PMID:39415693
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma. Reply., PMID:39383466
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma., PMID:39383465
Belantamab Mafodotin in Relapsed/Refractory AL Amyloidosis: Real-World Multi-Center Experience and Review of the Literature., PMID:39357511
Preclinical Evaluation of STI-8811, a Novel Antibody-Drug Conjugate Targeting BCMA for the Treatment of Multiple Myeloma., PMID:39292169
Drug-Related Keratitis: A Real-World FDA Adverse Event Reporting System Database Study., PMID:39287587
How to rescue a DreaMM deferred …., PMID:39276768
Belantamab mafodotin, pomalidomide, and dexamethasone for triple class exposed/refractory relapsed multiple myeloma: a subgroup analysis of the ALGONQUIN trial., PMID:39261451
Ocular adverse events associated with antibody-drug conjugates in oncology: a pharmacovigilance study based on FDA adverse event reporting system (FAERS)., PMID:39228525
Matching-adjusted indirect comparison of talquetamab vs selinexor-dexamethasone and vs belantamab mafodotin in patients with relapsed/refractory multiple myeloma., PMID:39226081
The Role of Monoclonal Antibodies in the Treatment of Myeloma Kidney Disease., PMID:39204135
Dynamics of microcyst-like epithelial changes associated with Belantamab mafodotin therapy in a patient with multiple myeloma-a case report., PMID:39112495
A Belantamab Mafodotin Revival in Multiple Myeloma Therapy., PMID:39083777
Belantamab-mafodotin-associated keratopathy., PMID:39003154
Corneal Toxicity in Patients Treated by BELANTAMAB MAFODOTIN: How to Improve and Facilitate Patients Follow-Up Using Refractive Shift?, PMID:38976493
Belantamab mafodotin concentration-QTc relationships in patients with relapsed or refractory multiple myeloma from the DREAMM-1 and -2 studies., PMID:38924122
Visualization of Keratopathy Associated With the Antibody-Drug Conjugate Belantamab Mafodotin Using Infrared Imaging in Patients With Multiple Myeloma., PMID:38900711
Belantamab mafodotin in triple-refractory multiple myeloma patients: A retro-prospective observational study in Italy., PMID:38895069
Successful treatment with belantamab mafodotin in a heavily pretreated patient with multiple myeloma and liver extramedullary disease., PMID:38850573
Belantamab Mafodotin, Pomalidomide, and Dexamethasone in Multiple Myeloma., PMID:38828951
Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma., PMID:38828933