Catalog No.
KDE16602
Description
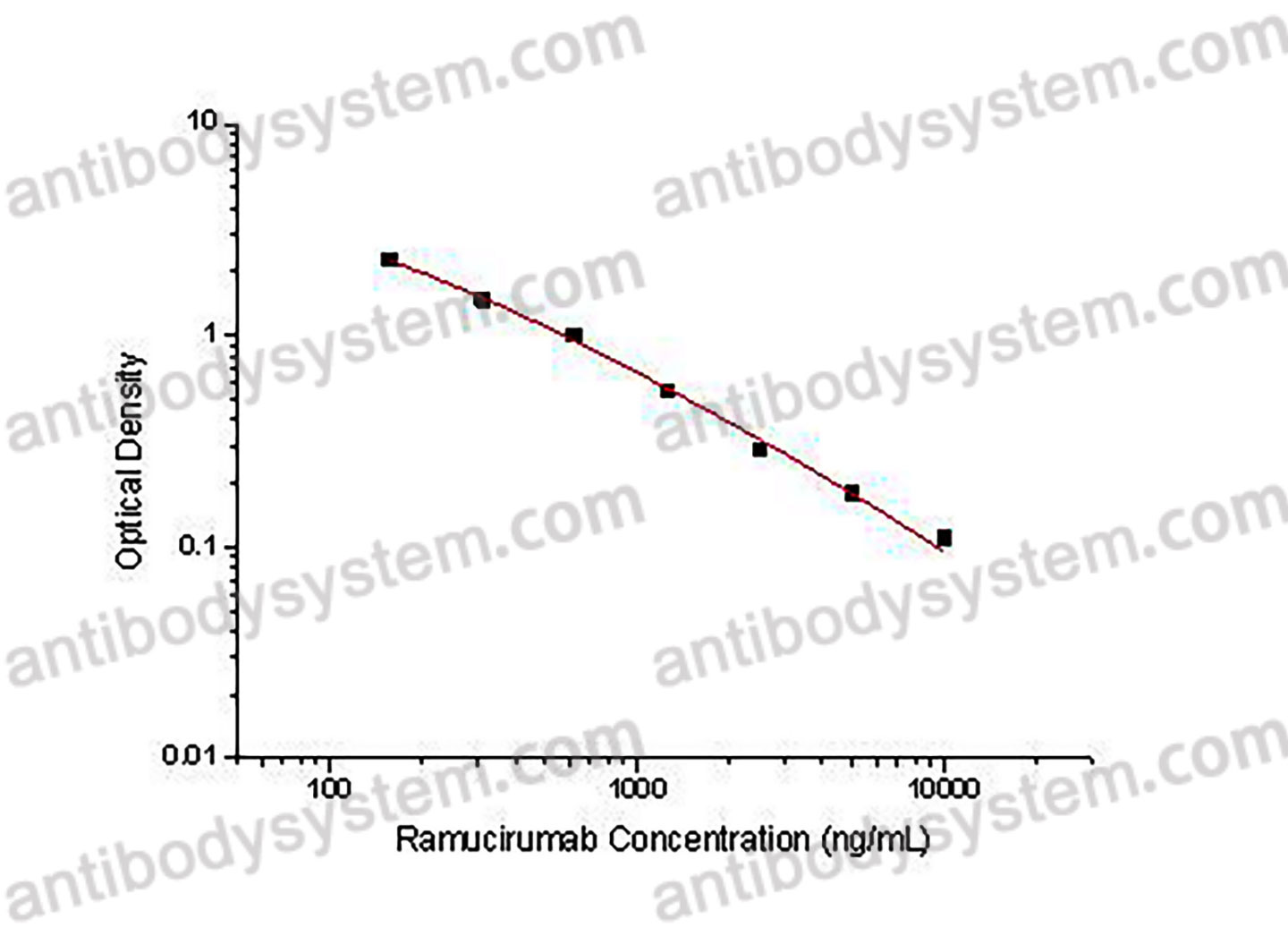
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD309 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Ramucirumab in the sample competitively binds to the pre-coated protein with biotin-labeled Ramucirumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Ramucirumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Ramucirumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
80.76 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5523.9
|
1358.9
|
272.9
|
5175.1
|
1340.9
|
309.4
|
|
Standard deviation
|
615.9
|
106.4
|
31.4
|
448.5
|
156.6
|
42.6
|
|
CV (%)
|
11.2
|
7.8
|
11.5
|
8.7
|
11.7
|
13.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
1121B, IMC-1121B, LY3009806, CAS: 15966-93-5
Background
Ramucirumab is a human monoclonal antibody (IgG1) against vascular endothelial growth factor receptor 2 (VEGFR2), a type II transmembrane tyrosine kinase receptor expressed on endothelial cells. By binding to VEGFR2, Ramucirumab prevents binding of its ligands (VEGF-A, VEGF-C, and VEGF-D), thereby preventing VEGF-stimulated receptor phosphorylation and downstream ligand-induced proliferation, permeability, and migration of human endothelial cells.
Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics., PMID:40508182
"Attenuated" Pulmonary Tumor Thrombotic Microangiopathy on Anti-Vascular Endothelial Growth Factor Treatment: A Case Report., PMID:40475293
Association Between Baseline Neutrophil Count and Febrile Neutropenia Following Docetaxel and Ramucirumab With Prophylactic Pegfilgrastim., PMID:40468685
Final Survival Outcomes With Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients With Untreated EGFR-Mutated Metastatic NSCLC: RELAY Japanese Subset., PMID:40458541
Trastuzumab Deruxtecan or Ramucirumab plus Paclitaxel in Gastric Cancer., PMID:40454632
EXPRESS: Advanced Therapies for Stomach Cancer., PMID:40448345
Randomized Phase III Trial of Ramucirumab Beyond Progression Plus Irinotecan in Patients With Ramucirumab-Refractory Advanced Gastric Cancer: RINDBeRG Trial., PMID:40408613
Comparison of dose rounding and drug vial optimization for reducing anticancer drug waste., PMID:40398488
Enhancing hepatocellular carcinoma treatment: synergistic cytotoxicity and mechanistic insights of Ramucirumab and 5-Azacytidine combination therapy., PMID:40397219
Real-world treatment and outcomes for EGFR WT advanced/metastatic non-squamous non-small cell lung cancer: pooled analysis from project LUMINATE-101., PMID:40338221
Therapeutic management of patients with advanced thymic malignancies: A review for clinicians., PMID:40334289
Maintenance Capecitabine Plus Ramucirumab After First-Line Chemotherapy in Patients With Advanced Esophagogastric Adenocarcinoma: Results From the Randomized PLATFORM Study., PMID:40330143
Unravelling Paclitaxel Resistance in Gastric Cancer: The Role of Small Extracellular Vesicles in Epithelial Mesenchymal Transition and Extracellular Matrix Remodelling., PMID:40282535
Thromboembolic and bleeding events associated with angiogenesis inhibitors in cancer patients., PMID:40246589
A disproportionality analysis of interstitial lung disease associated with drug therapy in spontaneous adverse event reports., PMID:40232264
Toxicity Profile and Efficacy of Docetaxel Following Paclitaxel- or Pemetrexed-Platinum Chemotherapy Alone or in Combination With Immune Checkpoint Inhibitors in NSCLC Patients: A Single Institution Retrospective Analysis., PMID:40220012
[A Case of Long-Term Prognosis of Advanced Rectosigmoid Cancer with Multiple Metastases Treated with Multidisciplinary Therapy]., PMID:40189763
S2303: phase II/III trial of paclitaxel + ramucirumab ± nivolumab in gastric and esophageal adenocarcinoma (PARAMUNE)., PMID:40155326
Ramucirumab and paclitaxel in second or greater lines of therapy in patients with HER2-positive gastroesophageal cancer: a single center study., PMID:40152313
Pairwise analysis of plasma cell-free DNA before and after palliative second-line paclitaxel plus ramucirumab treatment in patients with metastatic gastric cancer., PMID:40148708
Comparison of the prognostic effect of taxane regimens combined with ramucirumab before nivolumab for advanced gastric cancer., PMID:40095335
Successful Desensitization to Ramucirumab in Signet-Cell Gastric Adenocarcinoma., PMID:40079904
Nab-paclitaxel combined with cadonilimab (AK104) as second-line treatment for advanced gastric cancer: protocol for a phase II prospective, multicenter, single-arm clinical trial., PMID:40070819
Correction: Clinical Significance of Prior Ramucirumab Use on the Effectiveness of Nivolumab as the Third-Line Regimen in Gastric Cancer: A Multicenter Retrospective Study., PMID:40067648
Complete Remission with Nivolumab Monotherapy of Advanced Gastric Neuroendocrine Carcinoma., PMID:40024686
Drug-Induced Chylothorax During Chemotherapy With Ramucirumab and Paclitaxel for Advanced Gastric Cancer., PMID:39991332
In vitro Stability Study of a Panel of Commercial Antibodies at Physiological pH and Temperature as a Guide to Screen Biologic Candidate Molecules for the Potential Risk of In vivo Asparagine Deamidation and Activity Loss., PMID:39979532
[A Case of Multiple Brain Metastasis after Resection of Gastric Cancer with Adrenal Metastasis]., PMID:39948918
Monoclonal Antibodies and Small-Molecule Inhibitors Associated Osteonecrosis of Jaw: A Retrospective Pharmacovigilance Study., PMID:39922223
Phase II trial of nab-paclitaxel plus ramucirumab in combination with nivolumab for unresectable advanced or recurrent gastric cancer after progression on first-line treatment including fluoropyrimidine, platinum, and anti-PD-1/PD-L1 antibody (PADDLE)., PMID:39905373
A Case of Purpuric Papulopustular Eruption on the Extremities Developed During Erlotinib and Ramucirumab Combination Treatment, Resulting in Complete Regression Without Oral Prednisone or Discontinuing Chemotherapy., PMID:39881923
Efficacy of ramucirumab combined with erlotinib or osimertinib in untreated EGFR-mutated NSCLC patients with asymptomatic brain metastases: insights from molecular biomarkers in the RELAY-brain trial., PMID:39847172
A phase II study of ramucirumab and somatostatin analog therapy in patients with advanced neuroendocrine tumors., PMID:39834129
A non-interventional biomarker study in patients with adenocarcinoma of the lung treated with nintedanib plus docetaxel following progression on chemotherapy and/or immunotherapy: LUME-BioNIS., PMID:39830745
Development of treatment strategies for advanced HER2-positive gastric cancer: Insights from clinical trials., PMID:39805409
The Efficacy of FOLFIRI Plus Ramucirumab in Recurrent Colorectal Cancer Refractory to Adjuvant Chemotherapy with Oxaliplatin/Fluoropyrimidine-Including Biomarker Analyses., PMID:39796720
Immune Microenvironment and the Effect of Vascular Endothelial Growth Factor Inhibition in Hepatocellular Carcinoma., PMID:39769351
Circulating miR-23b-3p, miR-30e-3p, and miR-205-5p as Novel Predictive Biomarkers for Ramucirumab-Paclitaxel Therapy Outcomes in Advanced Gastric Cancer., PMID:39769259
Addressing the unmet need in NSCLC progression with advances in second-line therapeutics., PMID:39759220
[A Case of Pathological Complete Response after Nivolumab Therapy in Unresectable Advanced Gastric Cancer]., PMID:39721791
Alternating Administration of Osimertinib for Leptomeningitis and Docetaxel Plus Ramucirumab for Lung Adenocarcinoma Resistant to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Case Report., PMID:39721695
What is the optimal first-line regimen for advanced non-small cell lung cancer patients with epidermal growth factor receptor mutation: a systematic review and network meta-analysis., PMID:39695621
A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for ramucirumab., PMID:39656165
Advances in systemic therapy leading to conversion surgery for advanced hepatocellular carcinoma., PMID:39647858
Impact of ABCB1 single-nucleotide variants on early, extremely severe neutropenia induced by paclitaxel/nanoparticle albumin-bound paclitaxel in patients with gastric cancer., PMID:39622587
RELAY: Final Overall Survival for Erlotinib Plus Ramucirumab or Placebo in Untreated, EGFR-Mutated Metastatic NSCLC., PMID:39622410
S1701, A Randomized Phase 2 Trial of Carboplatin-Paclitaxel With and Without Ramucirumab in Patients With Locally Advanced, Recurrent, or Metastatic Thymic Carcinoma., PMID:39619274
A case of complete remission by cabozantinib as an end-line treatment for advanced hepatocellular carcinoma., PMID:39616585
Clinicopathological analysis of claudin 18.2 focusing on intratumoral heterogeneity and survival in patients with metastatic or unresectable gastric cancer., PMID:39615405
Systemic Therapy for Hepatocellular Carcinoma., PMID:39608951