Catalog No.
KDD72801
Description
PRINCIPLE OF THE ASSAY
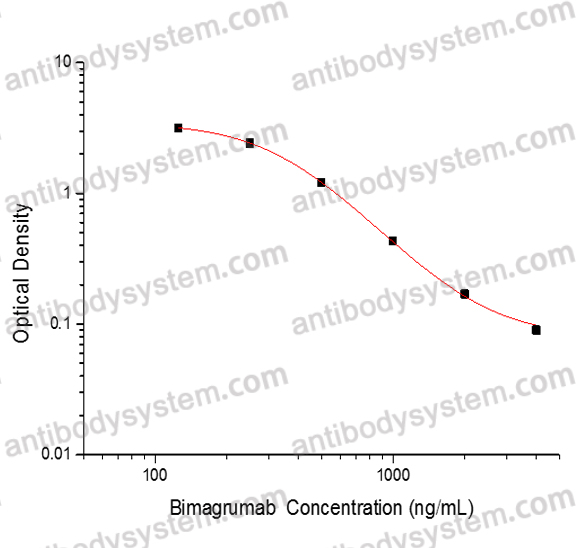
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human ACVR2A has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Bimagrumab in the sample competitively binds to the pre-coated protein with biotin-labeled Bimagrumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Bimagrumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Bimagrumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 4,000 ng/mL
Sensitivity
97.28 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1992.7
|
394.7
|
183.8
|
1817.1
|
458.8
|
255.4
|
|
Standard deviation
|
369.5
|
23.3
|
22.4
|
284.5
|
40.1
|
33.6
|
|
CV (%)
|
18.5
|
5.9
|
12.2
|
15.7
|
8.7
|
13.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
BYM338, CAS: 1356922-05-8
Background
Bimagrumab is a monoclonal anti-ActRIIA/IIB antibody that targets ActRIIA and ActRIIB simultaneously. Native ActRIIA and ActRIIB are both mediators of signaling from several TGFβ superfamily members such as activins, myostatin, and GDF11. Hence, pan-blockade by Bimagrumab achieves greater effect than a monoclonal antibody against either receptor alone, i.e. monotherapy with anti-ActRIIA antibody or anti-ActRIIB antibody. Bimagrumab has been explored as a therapy for sporadic inclusion body myositis, primary sarcopenia, and muscle wasting associated with chronic obstructive pulmonary disease.
Bimagrumab and sarcopenia., PMID:40471389
Current and Emerging Parenteral and Peroral Medications for Weight Loss: A Narrative Review., PMID:40422561
Nutrition support whilst on glucagon-like peptide-1 based therapy. Is it necessary?, PMID:40401903
New drugs for the treatment of obesity: do we need approaches to preserve muscle mass?, PMID:40320499
A Generic Detection Method for the Doping Control Analysis of Fc-Fusion Proteins and Monoclonal Antibodies in Equine Plasma., PMID:40033065
The Effect of Anti-Activin Receptor Type IIA and Type IIB Antibody on Muscle, Bone and Blood in Healthy and Osteosarcopenic Mice., PMID:39887865
Efficacy and safety of pharmacological treatments in inclusion body myositis: a systematic review., PMID:39843353
The impact of weight loss on fat-free mass, muscle, bone and hematopoiesis health: Implications for emerging pharmacotherapies aiming at fat reduction and lean mass preservation., PMID:39481534
Simultaneous detection of myostatin-targeting monoclonal antibodies in dried blood spots and plasma using liquid chromatography-tandem mass spectrometry with field asymmetric ion mobility spectrometry., PMID:39405785
Bimagrumab: an investigational human monoclonal antibody against activin type II receptors for treating obesity., PMID:39385353
Effect of Bimagrumab on body composition: a systematic review and meta-analysis., PMID:39251484
An open-label 16-week study of liraglutide in adolescents with obesity post-sleeve gastrectomy., PMID:39103247
What is the pipeline for future medications for obesity?, PMID:38302593
Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism., PMID:38218536
An update on peptide-based therapies for type 2 diabetes and obesity., PMID:36608818
Pharmacokinetics and Pharmacodynamics of Bimagrumab (BYM338)., PMID:36527600
Antibody Therapies in Autoimmune Inflammatory Myopathies: Promising Treatment Options., PMID:35394612
Drugs for Treating Obesity., PMID:34783910
Understanding of sarcopenia: from definition to therapeutic strategies., PMID:34537916
Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?, PMID:34518444
Blocking the activin IIB receptor with bimagrumab (BYM338) increases walking performance: A meta-analysis., PMID:34405505
Combined medical strategies for the management of type 2 diabetes mellitus and obesity in adults., PMID:34165376
Bimagrumab to improve recovery after hip fracture in older adults: a multicentre, double-blind, randomised, parallel-group, placebo-controlled, phase 2a/b trial., PMID:36098133
Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT., PMID:33597289
Challenges for Treatment Trials of Inclusion Body Myositis., PMID:33597288
Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial., PMID:33439265
Safety and pharmacokinetics of bimagrumab in healthy older and obese adults with body composition changes in the older cohort., PMID:33264516
Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial., PMID:33074327
Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen., PMID:32992744
Long-term safety and tolerability of bimagrumab (BYM338) in sporadic inclusion body myositis., PMID:32690797
Therapeutic potential of muscle growth promoters in a stress urinary incontinence model., PMID:32686522
[Late phase II/III study of BYM338 in patients with sporadic inclusion body myositis (RESILIENT): Japanese cohort data]., PMID:31761834
Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial., PMID:31397289
Endpoint choice for inclusion body myositis: a step too far?, PMID:31397280
Development of two complementary LC-HRMS methods for analyzing sotatercept in dried blood spots for doping controls., PMID:31218901
A review on the treatment of sporadic inclusion body myositis with Bimagrumab and Alemtuzumab., PMID:30238817
Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial., PMID:30095981
Commentary: Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy., PMID:29726548
Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes., PMID:29566437
Detection of the Human Anti-ActRII Antibody Bimagrumab in Serum by Means of Affinity Purification, Tryptic Digestion, and LC-HRMS., PMID:29226558
Reply to: New Hope for Sarcopenia., PMID:29148045
New Hope for Sarcopenia., PMID:29148043
Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy., PMID:29109273
Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy., PMID:28905498
The COPD Pipeline XXXII., PMID:28848893
Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study., PMID:28653345
Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals., PMID:28643356
Diagnosis and Management of Immune-Mediated Myopathies., PMID:28473041
Sarcopenia Trials in Specific Diseases: Report by the International Conference on Frailty and Sarcopenia Research Task Force., PMID:27883164
ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments., PMID:27462398