Catalog No.
KDD68901
Description
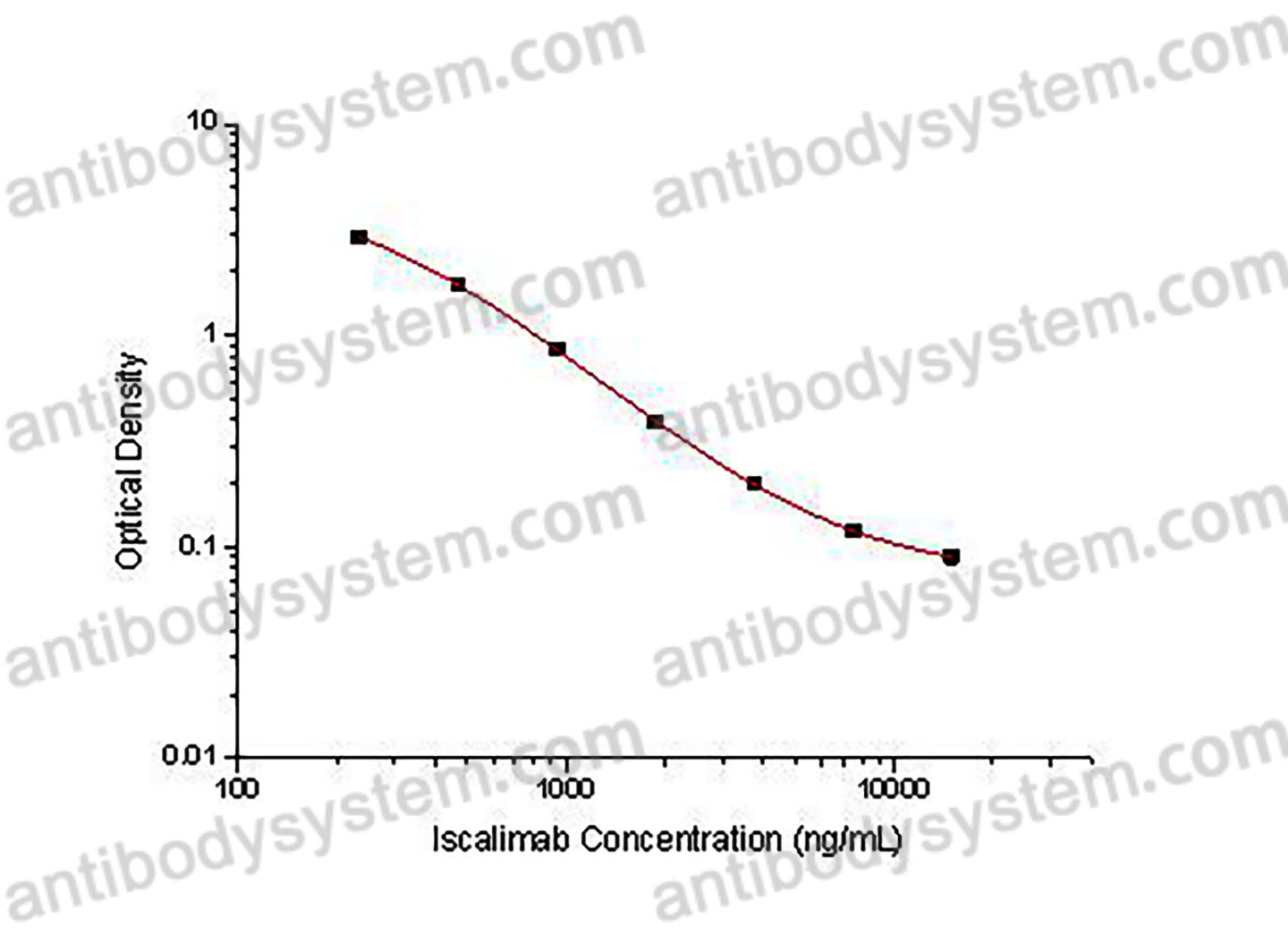
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD40 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Iscalimab in the sample competitively binds to the pre-coated protein with biotin-labeled Iscalimab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Iscalimab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Iscalimab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
144.20 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
8246.9
|
1312.9
|
658.1
|
8109.3
|
2488.5
|
1051.8
|
|
Standard deviation
|
1585.8
|
72.7
|
60.5
|
1217.6
|
162.8
|
114.9
|
|
CV (%)
|
19.2
|
5.5
|
9.2
|
15.0
|
6.5
|
10.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CFZ-533, NVP-CFZ533, OM11-62MF, CAS: 2031153-61-2
Background
Iscalimab is a new, fully human, monoclonal antibody preventing cluster of differentiation 40 (CD40) pathway signaling and activation of CD40+ cell types. In a recent multicenter, randomized control trial (NCT02217410), with the primary endpoint of non-inferiority on the composite endpoint, iscalimab therapy showed non-inferiority on a composite clinical endpoint, improved renal function, reduced risk for new onset diabetes and similar safety compared with tacrolimus. The analysis presented at the ATC included patients from this study that underwent either routine biopsies or biopsies as part of a follow-up protocol. The data was reviewed and scored by a blinded pathologist using the established Banff criteria and CADI. A CADI of 1 or less was considered as 'normal renal histology'. The average CADI at final biopsy was 1.6 ±0.6 for iscalimab and 5.1 ±0.8 for tacrolimus.
Iscalimab 600 mg: justified or excessive?, PMID:40318875
Iscalimab 600 mg: justified or excessive? - Authors' reply., PMID:40318874
Targeted immunotherapies for Graves' thyroidal & orbital diseases., PMID:40145088
Costimulation blockade: the next generation., PMID:39882641
[Iscalimab-New treatment option for patients with primary Sjögren's disease]., PMID:39738525
Infections in Sjögren's disease: a clinical concern or not?, PMID:39661562
New Developments and Therapeutic Drug Monitoring Options in Costimulatory Blockade in Solid Organ Transplantation: A Systematic Critical Review., PMID:39570574
Iscalimab Combined With Transient Tesidolumab Prolongs Survival in Pig-to-Rhesus Monkey Renal Xenografts., PMID:39185772
Safety and efficacy of subcutaneous iscalimab (CFZ533) in two distinct populations of patients with Sjögren's disease (TWINSS): week 24 results of a randomised, double-blind, placebo-controlled, phase 2b dose-ranging study., PMID:39096929
[Focus on Sjögren's syndrome - Diagnosis and treatment]., PMID:38781999
Efficacy and safety of iscalimab, a novel anti-CD40 monoclonal antibody, in moderate-to-severe myasthenia gravis: A phase 2 randomized study., PMID:37988976
Concurrent Ocular and Cerebral Toxoplasmosis in a Liver Transplant Patient Treated with Anti-CD40 Monoclonal Antibody., PMID:37545749
Single-cell transcriptomic analysis of renal allograft rejection reveals insights into intragraft TCR clonality., PMID:37227784
Single cell transcriptomic analysis of renal allograft rejection reveals novel insights into intragraft TCR clonality., PMID:36798151
Non-thionamide antithyroid drug options in Graves' hyperthyroidism., PMID:36740774
Tuberculosis dissemination in kidney transplant recipient treated with anti-CD40 monoclonal antibody: a case report., PMID:35986231
Immunosuppression Profile of CFZ533 (Iscalimab), a Non-Depleting Anti-CD40 Antibody, and the Presence of Opportunistic Infections in a Rhesus Monkey Toxicology Study., PMID:35730205
Current concepts regarding Graves' orbitopathy., PMID:35604323
Management of Graves' hyperthyroidism: present and future., PMID:35287535
Quantitative evaluation of drug efficacy in the treatment of myasthenia gravis., PMID:34821184
Targeted Therapy for Primary Sjögren's Syndrome: Where are We Now?, PMID:34731460
Precision Medicine in Graves' Disease: CD40 Gene Variants Predict Clinical Response to an Anti-CD40 Monoclonal Antibody., PMID:34149627
[Sjögren's syndrome: from diagnosis to treatment]., PMID:33689243
Novel Approaches for Immunosuppression in Graves' Hyperthyroidism and Associated Orbitopathy., PMID:33511082
Developments in immunosuppression., PMID:33332922
Blocking T cell co-stimulation in primary Sjögren's syndrome: rationale, clinical efficacy and modulation of peripheral and salivary gland biomarkers., PMID:33095146
Biologics in the treatment of Sjogren's syndrome, systemic lupus erythematosus, and lupus nephritis., PMID:33002950
Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren's syndrome: a multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study., PMID:38263652
Sjögren's syndrome: Old and new therapeutic targets., PMID:31831255
First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody., PMID:31647605
A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial., PMID:31512728
Nonclinical Safety Assessment of CFZ533, a Fc-Silent Anti-CD40 Antibody, in Cynomolgus Monkeys., PMID:30099540
Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody., PMID:29665205
Current status of costimulatory blockade in renal transplantation., PMID:27517137