Catalog No.
KDD46601
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD73 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Oleclumab in the sample competitively binds to the pre-coated protein with biotin-labeled Oleclumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Oleclumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Oleclumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
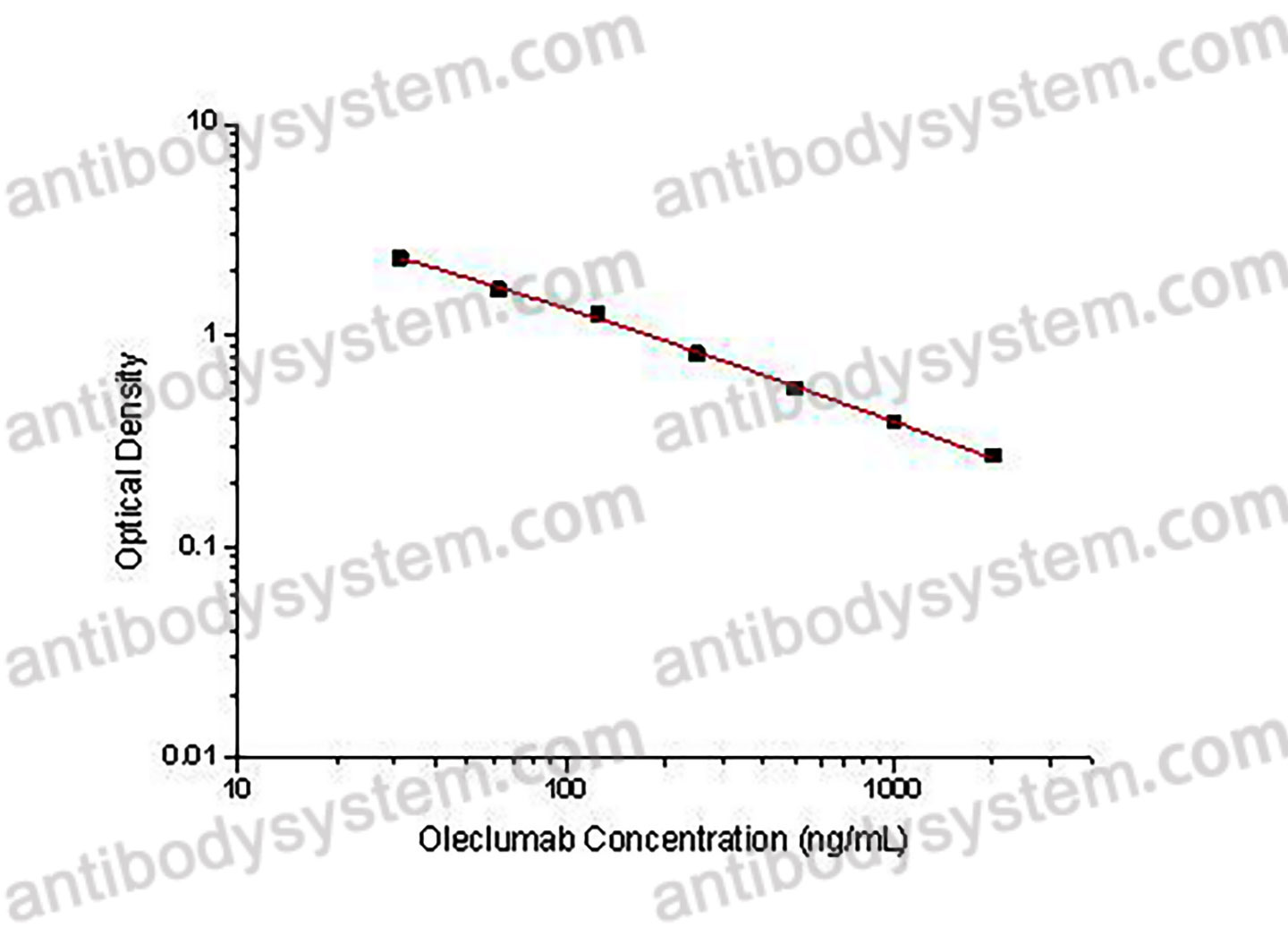
Range
31.25 - 2,000 ng/mL
Sensitivity
18.25 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2060.3
|
373.5
|
74.5
|
1709.3
|
354.3
|
83.4
|
|
Standard deviation
|
398.6
|
65.3
|
12.7
|
303.7
|
68.8
|
15.1
|
|
CV (%)
|
19.3
|
17.5
|
17.0
|
17.8
|
19.4
|
18.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MEDI-9447, , CAS: 1803176-05-7
Background
Oleclumab (as known as MEDI9447) is a human monoclonal antibody (mAb) being investigated for the treatment of various types of cancer like Solid Tumors, Pancreatic and Colorectal cancer. It is a monoclonal antibody against the ectoenzyme CD73 (cluster of differentiation 73), also known as 5'-nucleotidase (5'-NT; ecto-5'-nucleotidase; NT5E) with potential antineoplastic activity. This drug was developed by MedImmune, LLC and AstraZeneca plc. In contrast with many other cancer immunotherapy agents such as checkpoint inhibitors or T cell agonists, MEDI9447 drives changes in both myeloid and lymphoid infiltrating leukocyte populations within the tumor microenvironment. Changes include significant increases in CD8 effector cells and activated macrophages, as well as a reduction in the proportions of myeloid-derived suppressor cells (MDSC) and regulatory T lymphocytes. Furthermore, these changes correlate directly with responder and non-responder subpopulations within the arms of animal studies using syngeneic tumors. Data showing additive activity between MEDI9447 and other immune-mediated therapy antibodies demonstrates the importance of relieving adenosine-mediated immunosuppression within tumors.
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Adapting radiation therapy to immunotherapy: Delineation and treatment planning of pre-operative immune-modulating breast iSBRT in 151 patients treated in the randomized phase II Neo-CheckRay trial., PMID:40057199
Continuous replenishment of the dysfunctional CD8 T cell axis is associated with response to chemoimmunotherapy in advanced breast cancer., PMID:39983715
Peripheral blood leukocyte signatures as biomarkers in relapsed ovarian cancer patients receiving combined anti-CD73/anti-PD-L1 immunotherapy in arm A of the NSGO-OV-UMB1/ENGOT-OV30 trial., PMID:39887612
A Phase Ib/II Randomized Clinical Trial of Oleclumab with or without Durvalumab plus Chemotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma., PMID:39106081
COLUMBIA-1: a randomised study of durvalumab plus oleclumab in combination with chemotherapy and bevacizumab in metastatic microsatellite-stable colorectal cancer., PMID:39048638
PACIFIC-9: Phase III trial of durvalumab + oleclumab or monalizumab in unresectable stage III non-small-cell lung cancer., PMID:39023287
NSGO-OV-UMB1/ENGOT-OV30: A phase II study of durvalumab in combination with the anti-CD73 monoclonal antibody Oleclumab in patients with relapsed ovarian cancer., PMID:38943691
A phase 2 study of AZD4635 in combination with durvalumab or oleclumab in patients with metastatic castration-resistant prostate cancer., PMID:38430405
Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial., PMID:38351187
Efficacy and pharmacodynamic effect of anti-CD73 and anti-PD-L1 monoclonal antibodies in combination with cytotoxic therapy: observations from mouse tumor models., PMID:38206570
Author Correction: Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:38086864
First-in-human study of SBRT and adenosine pathway blockade to potentiate the benefit of immunochemotherapy in early-stage luminal B breast cancer: results of the safety run-in phase of the Neo-CheckRay trial., PMID:38056900
Paclitaxel plus carboplatin and durvalumab with or without oleclumab for women with previously untreated locally advanced or metastatic triple-negative breast cancer: the randomized SYNERGY phase I/II trial., PMID:37919269
Bispecific antibody CD73xEGFR more selectively inhibits the CD73/adenosine immune checkpoint on cancer cells and concurrently counteracts pro-oncogenic activities of CD73 and EGFR., PMID:37734877
Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial., PMID:37707791
A Novel Bispecific Antibody for EpCAM-Directed Inhibition of the CD73/Adenosine Immune Checkpoint in Ovarian Cancer., PMID:37509310
Tumor intrinsic and extrinsic functions of CD73 and the adenosine pathway in lung cancer., PMID:37033953
First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors., PMID:37016126
CD73 Inhibitor Oleclumab Plus Osimertinib in Previously Treated Patients With Advanced T790M-Negative EGFR-Mutated NSCLC: A Brief Report., PMID:36641093
Pharmacology, pharmacokinetics, and toxicity characterization of a novel anti-CD73 therapeutic antibody IBI325 for cancer immunotherapy., PMID:36587633
Safety, tolerability, pharmacokinetics, and antitumour activity of oleclumab in Japanese patients with advanced solid malignancies: a phase I, open-label study., PMID:36342599
Current challenges of unresectable stage III NSCLC: are we ready to break the glass ceiling of the PACIFIC trial?, PMID:35923929
Adenosine pathway inhibitors: novel investigational agents for the treatment of metastatic breast cancer., PMID:35575038
COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer., PMID:35452273
Bispecific antibody CD73xEpCAM selectively inhibits the adenosine-mediated immunosuppressive activity of carcinoma-derived extracellular vesicles., PMID:34464670
Neo-CheckRay: radiation therapy and adenosine pathway blockade to increase benefit of immuno-chemotherapy in early stage luminal B breast cancer, a randomized phase II trial., PMID:34362344