Catalog No.
KDD30801
Description
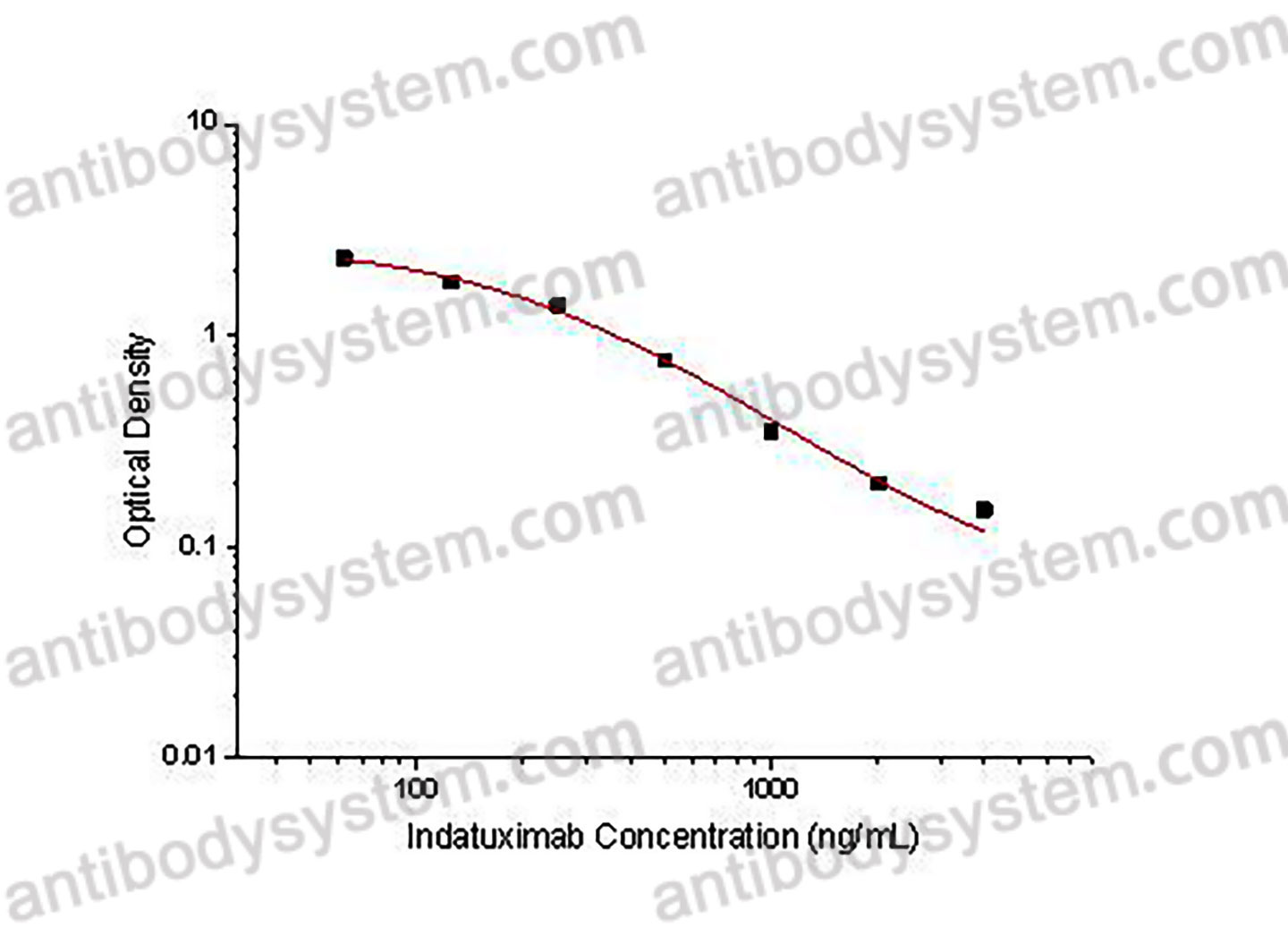
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD138 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Indatuximab in the sample competitively binds to the pre-coated protein with biotin-labeled Indatuximab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Indatuximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Indatuximab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
31.25 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
2056.9 |
468.6 |
120.2 |
2174.5 |
582.5 |
136.8 |
|
Standard deviation |
166.6 |
48.9 |
23.0 |
150.5 |
79.5 |
25.9 |
|
CV (%) |
8.1 |
10.4 |
19.1 |
6.9 |
13.7 |
18.9 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BT-062, nBT062-DM4, Indatuximab ravtansine(1238517-16-2)
Background
Indatuximab ravtansine (BT062) is an antibody-drug conjugate (ADC) based on a murine/human chimeric form of B-B4 (CD138-specific antibody), which is covalently conjugated to the maytansinoid drug DM4 via a disulfide bond-based linker. Maytansinoids are structural analogs of the cytotoxic agent maytansine, which has been evaluated in phase I and phase II clinical trials. They exhibit anti-mitotic activity by inducing metaphase arrest of dividing cells, causing cell death. Upon internalization of indatuximab ravtansine by target tumor cells, lysosomal processing of the disulfide linker generates a lysine metabolite which is reduced and S-methylated producing the lipophilic and cytotoxic metabolite, S-methyl-DM4. The mechanism of action of indatuximab ravtansine resembles that of trastuzumab emtansine, an antibody-drug conjugate indicated for HER2-positive metastatic breast cancer which uses the HER2-targeting properties of trastuzumab to deliver the cytotoxic DM1 within the cell by means of a stable linker. The intracellular drug delivery to target tumor cells can improve the therapeutic index of the cytotoxic drug and minimize exposure of normal tissue. Indatuximab ravtansine has previously been shown in vivo in multiple myeloma mouse xenograft models to significantly inhibit tumor growth and prolong host survival without any toxicity signals. Based on these preclinical results, a phase I clinical trial of indatuximab ravtansine demonstrated the first signs of clinical activity in patients with relapsed or refractory multiple myeloma without any toxicity signals. Furthermore, preliminary data from a phase I/IIa study in relapsed or refractory multiple myeloma indicated that indatuximab ravtansine is well tolerated even in a multiple-dose schedule and provided further evidence of clinical activity. Indatuximab ravtansine is being investigated as part of a treatment for multiple myeloma.