Catalog No.
KDD23001
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IFNAR1 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Anifrolumab in the sample competitively binds to the pre-coated protein with biotin-labeled Anifrolumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anifrolumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anifrolumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
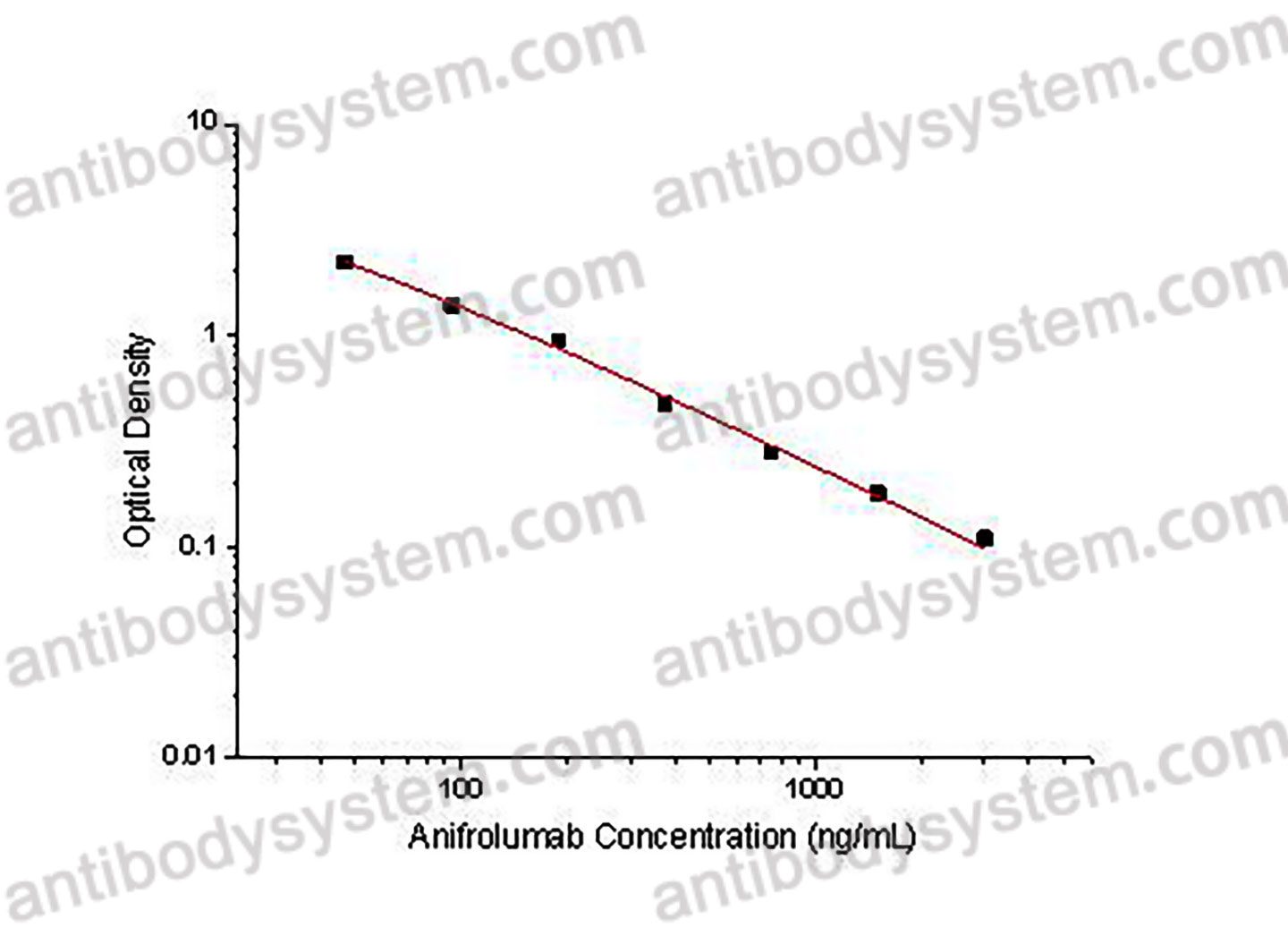
Range
46.88 - 3,000 ng/mL
Sensitivity
27.67 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1322.8
|
345.7
|
77.7
|
1614.7
|
364.6
|
73.9
|
|
Standard deviation
|
153.3
|
25.8
|
12.9
|
219.1
|
34.8
|
8.9
|
|
CV (%)
|
11.6
|
7.5
|
16.6
|
13.6
|
9.5
|
12.0
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MDX-1333, MEDI-546, CAS: 1326232-46-5
Background
Anifrolumab is one of three anti-type-1 interferon agents currently under investigation as a potential treatment for systemic lupus erythematosus (SLE). Initial in vivo studies observed higher levels of serum interferon (IFN) in patients with autoimmune disease as opposed to those of healthy controls. Further genetic analyses identified a consistent upregulation of IFN gene signatures in peripheral mononuclear cells of SLE patients. This signature was inducible by IFN, repressed with glucocorticoids, and possibly correlated with disease severity. Therefore, specific IFN gene signatures have been used in clinical trials as diagnostic and pharmacodynamic biomarkers in SLE.
[New treatments for cutaneous lupus]., PMID:40476397
Use of anifrolumab in a patient with chronic multirefractory Rowell's syndrome., PMID:40450451
Anifrolumab for refractory dermatomyositis: a case series., PMID:40454688
[[Translated article]]Real-World Experience with Anifrolumab in Cutaneous Lupus Erythematosus: Data from a Spanish Tertiary Referral Center., PMID:40446919
Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies., PMID:40430089
ERN ReCONNET-SLICC-SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations., PMID:40418946
Potential future biologic therapies for the treatment of vitiligo: focus on phase 2 and 3., PMID:40417830
Rapid response to anifrolumab in refractory systemic lupus erythematosus., PMID:40413848
Cutaneous lupus features specialized stromal niches and altered retroelement expression., PMID:40409678
Atypical infections during combination therapy with anifrolumab and other immunosuppressives in patients with lupus erythematosus: A case series., PMID:40405662
Anifrolumab for refractory discoid lupus: Two case reports of successful outcomes in Saudi Arabia., PMID:40388750
SLE-DAS enables an accurate definition of severe lupus disease activity: derivation and validation in a post hoc study of anifrolumab phase II and III studies., PMID:40379439
Progressive repigmentation of hypopigmented lesions in discoid lupus erythematosus with anifrolumab: A report of two cases., PMID:40378274
An Integrative Mechanistic Model of Type 1 IFN-Mediated Inflammation in Systemic Lupus Erythematosus., PMID:40364448
Adjunctive anifrolumab for recalcitrant discoid lupus erythematosus in the setting of systemic lupus erythematosus: A case report., PMID:40353109
Long-term effect of anifrolumab on patient-reported outcomes in systemic lupus erythematosus (TULIP-LTE): a randomised, placebo-controlled, phase 3 long-term extension trial., PMID:40324450
Combination Therapy With Voclosporin and Anifrolumab in Two Patients With Lupus Nephritis and Discoid Lupus Erythematosus., PMID:40322600
Anifrolumab in childhood-onset systemic lupus erythematosus: a promising option after belimumab failure., PMID:40312207
Anifrolumab in Refractory Dermatomyositis and Antisynthetase Syndrome., PMID:40297385
Hypertrophic lupus erythematosus hypertrophic lichen planus overlap responding to acitretin and anifrolumab., PMID:40276726
Anifrolumab in the management of lupus headaches: a case report., PMID:40242902
A novel treatment for refractory dermatomyositis: A systematic review of anifrolumab and dermatomyositis., PMID:40228662
Development of Diffuse Large B Cell Lymphoma in a Patient with Systemic Lupus Erythematosus within Two Years after the Initiation of Anifrolumab and Mycophenolate Mofetil Treatment., PMID:40222946
Rapid clinical and transcriptomic response to anifrolumab in refractory anti-TIF1γ-positive juvenile dermatomyositis., PMID:40221262
Flare of bullous lupus erythematosus in patients treated with anifrolumab for discoid lupus erythematosus: 2 cases., PMID:40206105
Drug-induced herpes zoster: a pharmacovigilance analysis of FDA adverse event reports from 2004 to 2024., PMID:40206093
RF - New Treatments in Cutaneous Lupus Erythematosus: Current and Future Perspectives., PMID:40204141
Bone marrow MxA staining reflects anifrolumab response in formerly treatment-refractory systemic lupus erythematosus with initial pancytopenia., PMID:40168663
Efficacy of anifrolumab as a first-line therapy for the treatment of thrombocytopenia in systemic lupus erythematosus: a case report., PMID:40153335
Anifrolumab for refractory cutaneous lupus lesions in pediatric systemic lupus erythematosus., PMID:40123095
Comparative efficacy and safety of anifrolumab for belimumab-experienced and biologic-naïve patients with systemic lupus erythematosus: Results from the Kyushu Collagen Disease Network for Systemic Lupus Erythematosus (KCDN-SLE) registry., PMID:40090613
Real-World Experience with Anifrolumab in Cutaneous Lupus Erythematosus: Data from a Spanish Tertiary Referral Center., PMID:40081476
[Translated article] Anifrolumab for the Treatment of Refractory Cutaneous Lupus Erythematosus: A Spanish Clinical Practice-Based Multicenter Study., PMID:40081475
Can Dermoscopy Be a Useful Follow-Up Tool in Patients with Discoid Lupus Treated with Anifrolumab?, PMID:40075770
Systemic lupus erythematosus: one year in review 2025., PMID:40072872
Efficacy and safety profile of anifrolumab in skin lesions of systemic lupus erythematosus., PMID:40052237
A focused report on IFN-1 targeted therapies for lupus erythematosus., PMID:40047795
Improvement in dermatomyositis-associated muscle disease with anifrolumab., PMID:40036364
Sustained Interferon Signature Suppression With Anifrolumab in a Patient With STING-Associated Vasculopathy with Onset in Infancy Refractory to JAK Inhibitor and Dazukibart Therapy., PMID:40007227
Current use and development of monoclonal antibodies for the treatment of systemic lupus erythematosus: a review., PMID:39958566
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials., PMID:39948001
Advances in Targeted Therapy for Systemic Lupus Erythematosus: Current Treatments and Novel Approaches., PMID:39940698
Characteristics and clinical outcomes of patients with systemic lupus erythematosus initiating anifrolumab in a real-world setting in Spain (AZAHAR study): an observational study protocol., PMID:39939126
Successful Treatment of Refractory Cutaneous Dermatomyositis With Anifrolumab., PMID:39925330
Characterization of T-bet expressing B cells in lupus patients indicates a putative prognostic and therapeutic value of these cells for the disease., PMID:39918986
Systemic lupus erythematosus-related macrophage activation syndrome inducing severe cytopenia successfully treated with anifrolumab: a case report., PMID:39907615
Correction to: Anifrolumab in Monogenic Lupus caused by TREX1 Mutation., PMID:39899156
Reduced organ damage accumulation in adult patients with SLE on anifrolumab plus standard of care compared to real-world external controls., PMID:39894690
Interferon activation in bone marrow long-lived plasma cells in systemic lupus erythematosus., PMID:39867907
Systemic Lupus Erythematous: Gene Polymorphisms, Epigenetics, Environmental, Hormonal and Nutritional Factors in the Consideration of Personalized Therapy., PMID:39866364