Catalog No.
KDD17401
Description
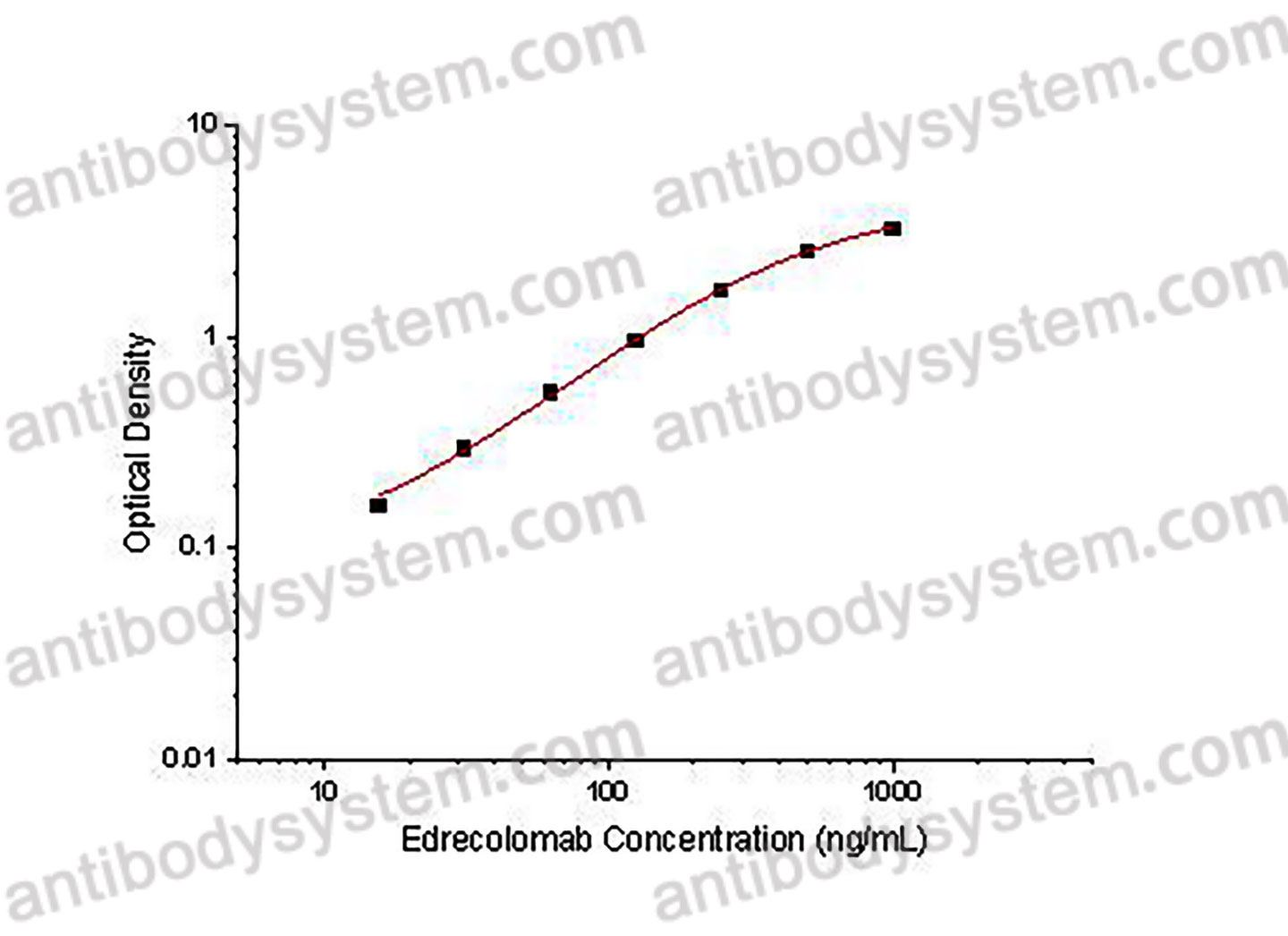
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human CD326 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Edrecolomab present is bound by the immobilized protein. After washing away any unbound substances, a HRP conjugated probe is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Edrecolomab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Edrecolomab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
15.63 - 1,000 ng/mL
Sensitivity
3.11 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
433.4
|
107.5
|
22.2
|
485.0
|
113.4
|
25.2
|
|
Standard deviation
|
36.4
|
7.6
|
1.2
|
29.7
|
4.0
|
3.5
|
|
CV (%)
|
8.4
|
7.0
|
5.2
|
6.1
|
3.5
|
14.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
17-1A, CAS: 156586-89-9
Background
Edrecolomab, also known as MAb17-1A, is a mouse-derived IgG2a monoclonal antibody targeted at the cell-surface glycoprotein EpCAM (17-1A). This drug was developed by Centocor in an attempt to prevent colorectal cancer and adenocarcinoma. Edrecolomab has been investigated in the clinical trials for the treatment of colon cancer. Edrecolomab was licensed as an adjuvant therapy for postoperative colorectal cancer by German authorities with the trade name Panorex in 1995. However, in 2004, researchers applied edrecolomab to the study of postoperative adjuvant therapy in patients with colorectal cancer. The results showed that edrecolomab adjuvant therapy can help Dukes'CCRC patients to restore the lack of immune response in the body, but the randomized trials of clinical efficacy were unsatisfactory. Then in 2005, Edrecolomab was studied in a phase 3 randomized trial of postoperative adjuvant therapy for colorectal cancer. However, the results showed that Edrecolomab did not improve overall survival or disease-free survival in patients with stage II colon cancer treated with postoperative adjuvant therapy. Subsequently, Edrecolomab in combination with fluorouracil (FU) was used to treat colorectal cancer in the III stage. However, the results showed that the addition of ED in the basic treatment of fluorouracil had no significant effect on OS. Edrecolomab also did not prolong patient survival in subsequent randomized trials. Although Edrecolomab has not shown very good results in previous studies of colorectal cancer, it is still a promising monoclonal antibody for the treatment of adenocarcinomas expressing EpCAM.
Impact of primary tumor side on the outcomes of patients with non-metastatic colon cancer; a patient-level pooled analysis of two clinical trials., PMID:29966444
Concise Review: Aggressive Colorectal Cancer: Role of Epithelial Cell Adhesion Molecule in Cancer Stem Cells and Epithelial-to-Mesenchymal Transition., PMID:29667344
EpCAM Immunotherapy versus Specific Targeted Delivery of Drugs., PMID:29329202
Antibody Based EpCAM Targeted Therapy of Cancer, Review and Update., PMID:29295696
Association Between Results of a Gene Expression Signature Assay and Recurrence-Free Interval in Patients With Stage II Colon Cancer in Cancer and Leukemia Group B 9581 (Alliance)., PMID:27432924
Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581., PMID:23530100
Marketed therapeutic antibodies compendium., PMID:22531442
Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803., PMID:21747089
Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581., PMID:21747085
[Progress of study on antitumor effects of antibody dependent cell mediated cytotoxicity--review]., PMID:21129296
Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies., PMID:21044305
FcγR polymorphisms and clinical outcome in colorectal cancer patients receiving passive or active antibody treatment., PMID:21042730
Adecatumumab: an anti-EpCAM monoclonal antibody, from the bench to the bedside., PMID:20426706
EpCAM as a target in cancer therapy., PMID:20385979
Letter to the editor: efficacy and safety of anti-Trop antibodies, R. Cubas, M. Li, C. Chen and Q. Yao, Biochim Biophys Acta 1796 (2009) 309-1., PMID:20079406
EpCAM: a potential antimetastatic target for gastric cancer., PMID:19941073
Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer., PMID:19273708
When wishful thinking leads to a misty-eyed appraisal: the story of the adjuvant colon cancer trials with edrecolomab., PMID:19273695
Yeast cell surface display system for determination of humoral response to active immunization with a monoclonal antibody against EpCAM., PMID:18321588
Influence of immunomodulatory drugs on the cytotoxicity induced by monoclonal antibody 17-1A and interleukin-2., PMID:17562330
EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges., PMID:17325709
Expression of EpCAM in uveal melanoma., PMID:17125516
Recent developments in colorectal cancer treatment by monoclonal antibodies., PMID:17049015
Drug evaluation: IGN-101--an anti-EpCAM murine antibody vaccine for cancer., PMID:16955700
Monoclonal and bispecific antibodies as novel therapeutics., PMID:16648969
Expression of epithelial-cell adhesion molecule (Ep-CAM) in small cell lung cancer as defined by monoclonal antibodies 17-1A and BerEP4., PMID:16227163
Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study., PMID:15933423
Lessons learned from the edrecolomab story: how a checkered past became a checkered flag for monoclonal antibodies in colorectal cancer therapy., PMID:15933417
[Anti-cancer monoclonal antibody]., PMID:15861718
Adjuvant therapy of colon cancer., PMID:15726511
The effect of Edrecolomab (Mo17-1A) or fluorouracil-based chemotherapy on specific immune parameters in patients with colorectal cancer. A comparative study., PMID:15713997
[Adjuvant therapy for colon cancer]., PMID:15497107
Anti-EpCAM monoclonal antibody (MAb17-1A) based treatment combined with alpha-interferon, 5-fluorouracil and granulocyte-macrophage colony-stimulating factor in patients with metastatic colorectal carcinoma., PMID:15289873
Monoclonal antibodies in the treatment of colorectal cancer., PMID:15177487
Monoclonal antibodies as therapeutic agents for cancer., PMID:15120666
Prevention of peritoneal carcinomatosis from human gastric cancer cells by adjuvant-type intraperitoneal immunotherapy in a SCID mouse model., PMID:14593230
EpCAM: A new therapeutic target for an old cancer antigen., PMID:14508099
Monoclonal antibodies in the treatment of cancer, Part 2., PMID:12966906
Immune changes in patients with colorectal cancer treated by adjuvant therapy with monoclonal antibody 17-1A: a pilot study., PMID:12962368
Monoclonal antibodies in the treatment of cancer, Part 1., PMID:12951753
A pilot study of edrecolomab (Panorex, 17-1A antibody) and capecitabine in patients with advanced or metastatic adenocarcinoma., PMID:12743982
Adjuvant therapy for colon cancer in the new millenium., PMID:12705552
Edrecolomab in the adjuvant treatment of colorectal carcinoma., PMID:12517502
Edrecolomab in the adjuvant treatment of colorectal carcinoma., PMID:12517501
Highlights from: 38th annual meeting of the American Society of Clinical Oncology., PMID:12453319
Edrecolomab (Panorex) as adjuvant therapy for stage II colon cancer., PMID:12445374
25th European Society for Medical Oncology Congress. Hamburg, Germany. October 13-17, 2000., PMID:12445373
Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study., PMID:12241873
Sequential immunochemotherapy and edrecolomab in the adjuvant therapy of breast cancer: reduction of 17-1A-positive disseminated tumour cells., PMID:12176782
In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment., PMID:12115595