Catalog No.
KDD16101
Description
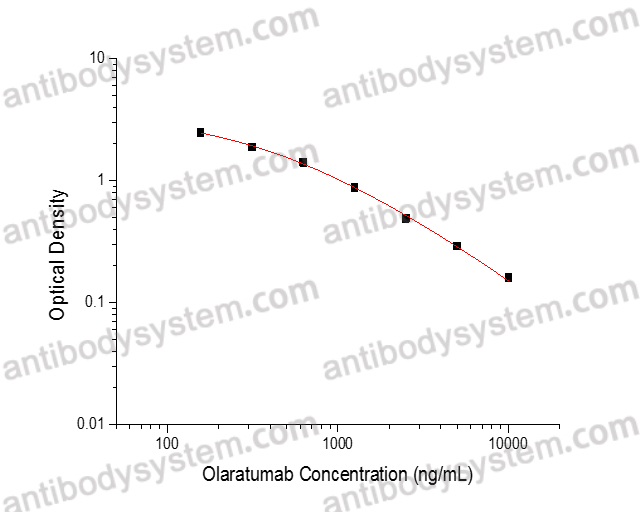
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD140a has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Olaratumab in the sample competitively binds to the pre-coated protein with biotin-labeled Olaratumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Olaratumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Olaratumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
112.17 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5830.6
|
1331.4
|
305.7
|
6891.0
|
1451.8
|
310.8
|
|
Standard deviation
|
478.4
|
117.8
|
41.8
|
1025.4
|
194.1
|
58.7
|
|
CV (%)
|
8.2
|
8.8
|
13.7
|
14.9
|
13.4
|
18.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
3G3, IMC-3G3, LY3012207, CAS: 1024603-93-7
Background
Olaratumab (IMC-3G3) is a fully human IgG1 monoclonal antibody developed by Eli Lilly and Company. It selectively binds the external domain of human platelet-derived growth factor receptor (PDGFR). With the trade name Lartruvo, olaratumab has been approved by USA, Canada, and European Union to use in combination with doxorubicin for the treatment of adults with advanced soft-tissue sarcoma (STS) who cannot be cured by cancer surgery or radiation therapy, and who have not been previously treated with doxorubicin.
From Growth Factors to Structure: PDGF and TGF-β in Granulation Tissue Formation. A Literature Review., PMID:40495632
Real-world survival outcomes and MDM2 prevalence in US patients with metastatic dedifferentiated liposarcoma., PMID:40468683
What are the Optimal Systemic Treatment Options for Rhabdomyosarcoma?, PMID:38750399
Results of a Randomized, Double-Blind, Placebo-Controlled, Phase 1b/2 Trial of Nabpaclitaxel + Gemcitabine ± Olaratumab in Treatment-Naïve Participants with Metastatic Pancreatic Cancer., PMID:38611000
Best Overall Response-Associated Signature to Doxorubicin in Soft Tissue Sarcomas: A Transcriptomic Analysis from ANNOUNCE., PMID:38536068
Randomized Phase 2 Clinical Trial of Olaratumab in Combination with Gemcitabine and Docetaxel in Advanced Soft Tissue Sarcomas., PMID:37835565
Case report: The activity of multi-kinase VEGF inhibitor, Pazopanib, in metastatic undifferentiated round cell sarcomas harboring EWSR1::CREM fusion: clinicopathological series of two cases and literature review., PMID:37829338
Results of an Open-label, Phase Ia/b Study of Pembrolizumab plus Olaratumab in Patients with Unresectable, Locally Advanced, or Metastatic Soft-Tissue Sarcoma., PMID:37382656
Doxorubicin combined with Trabectedin in systemic first-line M+/recurrent leiomyosarcoma patients., PMID:37222199
PDGFRα monoclonal antibody: Assessment of toxicity in juvenile mice administered a murine surrogate antibody of olaratumab., PMID:36916488
Quality of life of patients with soft tissue sarcoma treated with doxorubicin in the ANNOUNCE phase III clinical trial., PMID:35547106
Fooled by Randomness. The Misleading Effect of Treatment Crossover in Randomized Trials of Therapies with Marginal Treatment Benefit., PMID:34919008
Osteosarcoma Patient-derived Orthotopic Xenograft (PDOX) Models Used to Identify Novel and Effective Therapeutics: A Review., PMID:34848441
Olaratumab-induced Biomarker Modulation in Sarcomas-Response., PMID:34607902
Olaratumab-induced Biomarker Modulation in Sarcomas-Letter., PMID:34607901
Olaratumab's failure in soft tissue sarcoma., PMID:34349891
Primary Intracranial Leiomyosarcoma Secondary to Glioblastoma: Case Report and Literature Review., PMID:34094927
Interim Analysis of the Phase II Study: Noninferiority Study of Doxorubicin with Upfront Dexrazoxane plus Olaratumab for Advanced or Metastatic Soft-Tissue Sarcoma., PMID:33766818
Modern multimodality management of patients with caval leiomyosarcoma: New treatment paradigms and potential molecular insights., PMID:33650695
Response to the combination use of pazopanib with olaratumab in a patient with lung-metastatic embryonal rhabdomyosarcoma: a case report., PMID:33569329
Expression and prognostic significance of PDGF ligands and receptors across soft tissue sarcomas., PMID:33524869
Phase 1 trial of olaratumab monotherapy and in combination with chemotherapy in pediatric patients with relapsed/refractory solid and central nervous system tumors., PMID:33474828
Circulating Tumor Cells and Biomarker Modulation with Olaratumab Monotherapy Followed by Olaratumab plus Doxorubicin: Phase Ib Study in Patients with Soft-Tissue Sarcoma., PMID:33177152
Accelerated Approval of Anticancer Drugs: Lessons Learned From the Example of Olaratumab., PMID:33147355
Angiosarcomas of Primary Gynecologic Origin - A Case Series and Review of the Literature., PMID:32988901
Good and sustained response to pembrolizumab and pazopanib in advanced undifferentiated pleomorphic sarcoma: a case report., PMID:32670543
Real-world experience with doxorubicin and olaratumab in soft tissue sarcomas in England and Northern Ireland., PMID:32391141
Effect of Doxorubicin Plus Olaratumab vs Doxorubicin Plus Placebo on Survival in Patients With Advanced Soft Tissue Sarcomas: The ANNOUNCE Randomized Clinical Trial., PMID:32259228
Treatment for Advanced Soft Tissue Sarcoma: An Informative Neutral Trial., PMID:32259216
Doxorubicin and Olaratumab Versus Doxorubicin, Ifosfamide, and Mesna for Treatment of Advanced Soft Tissue Sarcomas., PMID:32235164
Metastatic Hepatic Epithelioid Hemangioendothelioma Treated with Olaratumab: A Falling Star Rising?, PMID:32161464
Novel Aspects of Genetics, Molecular Biology and Clinical Oncology of Sarcomas., PMID:32075391
Combination therapy with Olaratumab/doxorubicin in advanced or metastatic soft tissue sarcoma -a single-Centre experience., PMID:31996176
Pharmacokinetics of doxorubicin following concomitant intravenous administration of olaratumab (IMC-3G3) to patients with advanced soft tissue sarcoma., PMID:31821732
Advances in soft-tissue sarcoma - There are no mistakes, only lessons to learn!, PMID:31807494
Time to Review Authorisation and Funding for New Cancer Medicines in Europe? Inferences from the Case of Olaratumab., PMID:31696433
Relevance of Anti-Galactose-α-1,3-Galactose Antibodies in the Era of Monoclonal Antibodies., PMID:31693451
Negative phase III trials announce the need for biomarkers in sarcoma., PMID:31675613
Revocation of the conditional marketing authorisation of a cancer medicine: The olaratumab experience., PMID:31655357
Olaratumab plus anthracyline in advanced/metastatic soft tissue sarcoma : Data of real-world utilization in Austria., PMID:31620878
Multiple systemic treatment options in a patient with malignant tenosynovial giant cell tumour., PMID:31567307
A pilot study of first-line olaratumab, doxorubicin and ifosfamide in patients with metastatic soft tissue sarcoma., PMID:31410509
Assessment of the platelet-derived growth factor receptor alpha antibody olaratumab in a panel of patient-derived soft tissue sarcoma xenografts., PMID:31331295
Olaratumab administered in two cases of phyllodes tumour of the breast: end of the beginning?, PMID:31321082
The Combination of Olaratumab with Doxorubicin and Cisplatinum Regresses a Chemotherapy-Resistant Osteosarcoma in a Patient-Derived Orthotopic Xenograft Mouse Model., PMID:31299622
Incidence and Management of Olaratumab Infusion-Related Reactions., PMID:31268811
Olaratumab failure in sarcomas: what are the lessons learned?, PMID:31254941
American Society of Clinical Oncology (ASCO) - 55th Annual Meeting (May 31-June 4, 2019 - Chicago, Illinois, USA)., PMID:31250844
ANNOUNCE prompts questions over the Accelerated Approval process., PMID:31249400