Catalog No.
KDD15101
Description
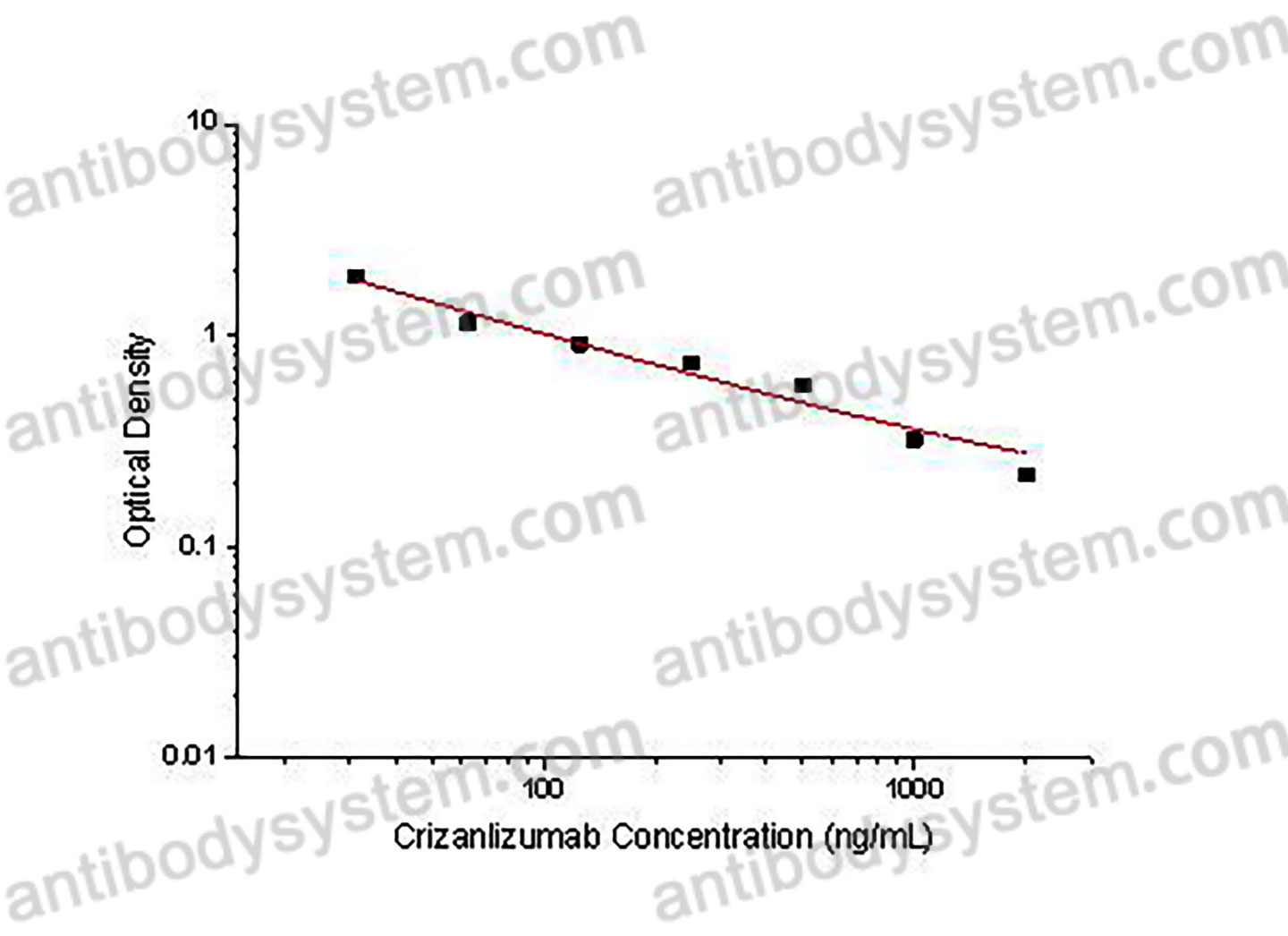
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD62P has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Crizanlizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Crizanlizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Crizanlizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Crizanlizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
3.94 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
933.2
|
190.7
|
35.3
|
1383.7
|
209.0
|
47.5
|
|
Standard deviation
|
125.2
|
34.0
|
6.9
|
274.6
|
25.5
|
9.4
|
|
CV (%)
|
13.4
|
17.8
|
19.6
|
19.8
|
12.2
|
19.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
SelG1, CAS: 1690318-25-2
Background
Crizanlizumab is a humanized monoclonal antibody directed against P-selectin that showed effectiveness in preventing VOC induced pain crises. Median rate of pain crises was 1.63 in crizanlizumab treated group versus 2.998 in placebo group. Furthermore, the median time to crisis was 4.07 months with crizanlizumab compared to 1.38 in the placebo group. The benefits of treatment held true within subgroups including those with a high number of VOC prior to treatment and those with more severe genotype. The drug is FDA approved to reduce the frequency of VOC in adults and pediatric patients with SCD aged 16 years and older.
ADORE: an open platform study of ruxolitinib in combination with other novel therapies in patients with myelofibrosis., PMID:40334082
Evidence and gaps in clinical outcomes of novel pharmacologic therapies for sickle cell disease: A systematic literature review highlighting insights from clinical trials and real-world studies., PMID:40307078
Clinically relevant clot resolution via a thromboinflammation-on-a-chip., PMID:40175551
Impact of Different Definitions of Vaso-Occlusion on Efficacy Assessments in Sickle Cell Disease Clinical Trials., PMID:40146367
Now where do we STAND with crizanlizumab?, PMID:40088923
Crizanlizumab with or without hydroxyurea in patients with sickle cell disease (STAND): primary analyses from a placebo-controlled, randomised, double-blind, phase 3 trial., PMID:40088922
Accelerated drug approvals and patient trust: impact of voxelotor and crizanlizumab for sickle cell disease., PMID:40085957
A critique review of fetal hemoglobin modulators through targeting epigenetic regulators for the treatment of sickle cell disease., PMID:40057227
A review on disease modifying pharmacologic therapies for sickle cell disease., PMID:39828781
A contemporary review of the management strategies for sickle cell disease related ischaemic and stuttering priapism., PMID:39709509
Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study., PMID:39680465
Practical guide for disease-modifying medication management of children and adolescents with sickle cell disease., PMID:39644011
Novel Pathway and Recent Advances for Targeting Sickle Cell Anemia through Novel Drug Delivery System., PMID:39629577
Targeting P-selectin and interleukin-1β in mice with sickle cell disease: effects on vaso-occlusion, liver injury and organ iron deposition., PMID:39568425
Promising role of voxelotor in managing sickle cell disease in children: a narrative review., PMID:39533724
The Current Role of Hydroxyurea in the Treatment of Sickle Cell Anemia., PMID:39518543
Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study., PMID:39497751
Real-World Evidence of Crizanlizumab Showing Reductions in Vaso-Occlusive Crises and Opioid Usage in Sickle Cell Disease., PMID:39473076
Gene Therapies for Sickle Cell Disease., PMID:39391326
Advancing life: innovative approaches to enhance survival in sickle cell anemia patients., PMID:39359845
Newer Modalities and Updates in the Management of Sickle Cell Disease: A Systematic Review., PMID:39286637
Budget Impact of Disease-Modifying Treatments and a CRISPR Gene-Edited Therapy for Sickle Cell Disease., PMID:39134876
Exploring the genetic mechanisms: SELP gene's contribution to alleviating vaso-occlusive crisis in sickle cell disease., PMID:39079562
Current and emerging drug treatment strategies to tackle sickle cell anemia., PMID:38988318
The enigma of sickle cell hepatopathy: Pathophysiology, clinical manifestations and therapy., PMID:38978231
Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study., PMID:38950286
Potential efficacy of crizanlizumab in treating priapism in sickle cell disease: A case report., PMID:38736574
Clinical Practice Patterns in Sickle Cell Disease Treatment: Disease-modifying and Potentially Curative Therapies., PMID:38718300
Cost-effectiveness of l-glutamine versus crizanlizumab for adults with sickle cell disease: model focused on reducing pain episode costs from Qatar's healthcare perspective., PMID:38711465
Sickle Cell Disease in Brazil: Current Management., PMID:38663998
Expert consensus on the management of infusion-related reactions (IRRs) in patients with sickle cell disease (SCD) receiving crizanlizumab: a RAND/UCLA modified Delphi panel., PMID:38642304
Spanish registry of hemoglobinopathies and rare anemias (REHem-AR): demographics, complications, and management of patients with β-thalassemia., PMID:38519604
Plant-Produced Therapeutic Crizanlizumab Monoclonal Antibody Binds P-Selectin to Alleviate Vaso-occlusive Pain Crises in Sickle Cell Disease., PMID:38491245
Crizanlizumab and sickle cell disease: When should medications have their approval status revoked?, PMID:38409818
Recurrent Nontraumatic Subgaleal Hematomas in a Pediatric Patient With Sickle Cell Disease., PMID:38408160
An update review of new therapies in sickle cell disease: the prospects for drug combinations., PMID:38344818
Casgevy and Lyfgenia: Two gene therapies for sickle cell disease., PMID:38212256
Real-world experience of patients with sickle cell disease treated with crizanlizumab., PMID:38073007
Using disease-modifying therapies in sickle cell disease., PMID:38066905
Use of Disease-Modifying Treatments in Patients With Sickle Cell Disease., PMID:37991760
Red Blood Cells as Therapeutic Target to Treat Sickle Cell Disease., PMID:37975291
Comparative pharmacovigilance assessment of adverse events associated with the use of hydroxyurea, L-glutamine, voxelotor, and crizanlizumab in sickle cell disease., PMID:37950855
Sickle cell disease: combination new therapies vs. CRISPR-Cas9 potential and challenges - review article., PMID:37867187
Crizanlizumab in sickle cell disease., PMID:37850353
Successful Treatment of SCD-Related Priapism With Crizanlizumab: A Case Series., PMID:37731262
Advancements in Sickle Cell Disease (SCD) Treatment: A Review of Novel Pharmacotherapies and Their Impact on Patient Outcomes., PMID:37664319
The Role of Non-genetic Therapies to Reduce the Incidence of Sickle Cell Crisis: A Systematic Review., PMID:37664256
P-Selectin de-ACTIVation in COVID-19: What Have We Learned?, PMID:37523764