Catalog No.
KDD12602
Description
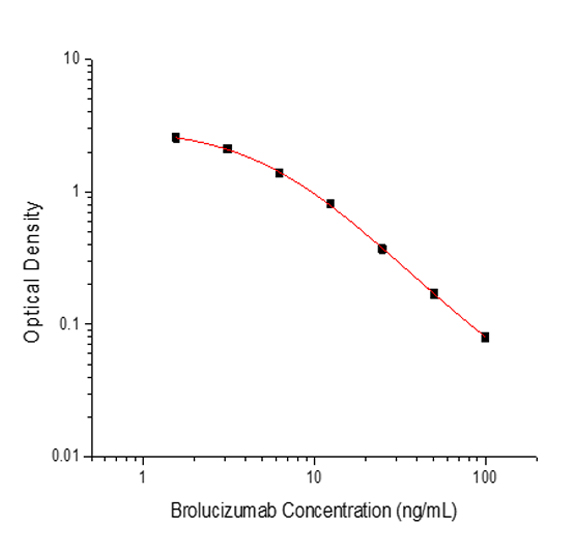
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human VEGFA has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Brolucizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Brolucizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Brolucizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Brolucizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.75 ng/mL
Precision
CV<20%
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
ESBA-1008, ESBA1008, RTH258, CAS: 1531589-13-5
Background
Brolucizumab is the first and only single-chain variable fragment (scFv) targeting vascular endothelial growth factor (VEGF)-A. It has a smaller molecular weight (26 kDa). The mAb is undergoing evaluation as a treatment for neovascular age-related macular degeneration (nAMD). In June 2017, Novartis announced that the primary efficacy endpoint of non-inferiority to aflibercept (EYLEA®) in mean change in best-corrected visual acuity from baseline to week 48 was met in the Phase 3 HAWK (NCT02307682) and HARRIER (NCT02434328) studies, which included more than 1,800 patients. The two studies compared the efficacy and safety of intravitreal injections of 6 mg brolucizumab or 3 mg brolucizumab (HAWK study only) versus 2 mg aflibercept in patients with nAMD. Of patients receiving 6 mg brolucizumab, 57% (HAWK) and 52% (HARRIER) were maintained exclusively on an every 12 week dosing interval immediately following the loading phase and continuing through week 48.54 Key secondary endpoints were also met. Novartis is developing a competitive, low cost of goods formulation, and expects to complete the PK study with antibody derived from the final manufacturing process to enable filing in 2018.
Real-world outcomes, including intraocular inflammation, after intravitreal brolucizumab for diabetic macular edema., PMID:40500587
Usage of brolucizumab as treatment for wet age-related macular degeneration (AMD) and polypoidal choroidal vasculopathy (PCV): A narrative review., PMID:40489855
Brolucizumab clinical and safety outcomes in a neovascular age-related macular degeneration national database: Fight Retinal Blindness Spain (FRB Spain)., PMID:40473932
TALON Phase IIIb Study: 32 Week Primary Results of Brolucizumab Using Treat and Extend for Neovascular Age Related Macular Degeneration., PMID:40466770
Association between intravitreal anti-vascular endothelial growth factor agents and hypertension: a meta-analysis., PMID:40456283
Real-World Outcomes of Brolucizumab Treatment in Japanese Patients with Neovascular Age-Related Macular Degeneration: A 12-Month, Multicenter Study., PMID:40434532
Authors' response: "Brolucizumab-associated intraocular inflammation in Indian patients by VRSI study group"., PMID:40434471
Comment on "Brolucizumab-associated intraocular inflammation in Indian patients by VRSI study group"., PMID:40434470
Brolucizumab results and adverse visual outcomes study: A single-center real-world Indian experience., PMID:40434468
Successful Response to Intravitreal Faricimab Injections in a Case of Neovascular Age-Related Macular Degeneration in Vitrectomized Eyes., PMID:40400877
The effect of brolucizumab on diabetic macular edema and ischemia; a real-world analysis., PMID:40398452
Drug-induced Vasculitis and Choroiditis after Brolucizumab., PMID:40383437
Clinical implication of aberrant choroidal feeding artery in type 1 macular neovasculopathy., PMID:40335557
Real-world safety and efficacy of Anti-VEGF treatment in Brazil., PMID:40298749
Intraocular Inflammation, Safety Events, and Outcomes After Intravitreal Injection of Ranibizumab, Aflibercept, Brolucizumab, Abicipar Pegol, and Faricimab for nAMD., PMID:40271423
Proactive Post-Injection Monitoring in Brolucizumab Therapy: A Study on Intraocular Inflammation and Treatment Outcomes., PMID:40270623
Comparison of one-year outcomes between aflibercept and brolucizumab for treatment-naïve pachychoroid neovasculopathy., PMID:40259096
Two-Years Real-World Experience of a Tertiary Center with Intravitreal Brolucizumab Switch for Treatment of Exudative Neovascular Age-Related Macular Degeneration., PMID:40249498
Safety and effectiveness of brolucizumab in patients with neovascular age-related macular degeneration: A phase IV study from India., PMID:40243051
Comparative efficacy of intravitreal anti-VEGF therapy for neovascular age-related macular degeneration: A systematic review with network meta-analysis., PMID:40241463
Reinjection in Patients with Intraocular Inflammation Development after Intravitreal Brolucizumab Injection., PMID:40235102
Three-year visual outcomes after brolucizumab in patients with neovascular age-related macular degeneration., PMID:40229766
Novel Resolution of Multilayered Pigment Epithelial Detachment Lamellae Following Brolucizumab Treatment-A Case Report., PMID:40224928
Molecular Dynamic Stability Study of VEGF Inhibitor in Patients with Bladder Cancer., PMID:40223853
Efficacy of Aflibercept (8 mg) for Diabetic Macular Edema in Vitrectomized Eyes Refractory to the Other Anti-VEGF Drug Therapies: A Report of Three Cases., PMID:40206271
Protocol for a Randomized Controlled Trial Comparing Intravitreal Brolucizumab with Placebo for Chronic Central Serous Chorioretinopathy with Persistent Macular Fluid Without Choroidal Neovascular Membrane - BRICS Report #1., PMID:40151914
Dynamics of Inflammatory Factors in Aqueous Humor During Brolucizumab Treatment for Age-Related Macular Degenerations: A Case Series., PMID:40142183
Comparison Between Intravitreal Anti-Vascular Endothelial Growth Factor Monotherapy and Vitrectomy in Age-Related Macular Degeneration with Large Submacular Hemorrhages., PMID:40094950
Cost-effectiveness analysis of bispecific antibody faricimab for treatment of neovascular age-related macular degeneration and diabetic macular edema in Japan., PMID:40078048
Cost-Effectiveness and Budget Impact Analysis of the Use of Faricimab in Diabetic Macular Edema and Neovascular Age-Related Macular Degeneration in Colombia., PMID:40051780
Increased Risk of Retinal Vasculitis May Be Associated with Aflibercept 8 mg: A Pharmacovigilance Analysis of the FAERS Database., PMID:40048264
Efficacy, Safety, and Injection Frequency with Novel Aflibercept 8 mg in Neovascular Age-Related Macular Degeneration: A Comparison with Existing Anti-VEGF Regimens Using a Bayesian Network Meta-Analysis., PMID:39994103
Budget Impact Analysis of Intravitreal Injections Used to Treat Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in the Dubai Healthcare System., PMID:39978290
Efficacy and safety of brolucizumab every 6 weeks induction therapy for neovascular age-related macular degeneration., PMID:39962248
Changes in Aqueous Humor Cytokine Profile Following Intravitreal Brolucizumab Injection., PMID:39959882
Faricimab efficacy in a poorly responsive case of Polypoidal Choroidal Vasculopathy (PCV): A case report., PMID:39936369
Comparative Efficacy of Brolucizumab and Aflibercept in Polypoidal Choroidal Vasculopathy: A Systematic Review and Meta-Analysis., PMID:39917144
Predicting benefit from adjuvant therapy with corticosteroids in community-acquired pneumonia: a data-driven analysis of randomised trials., PMID:39892408
Adaptive Optics Ophthalmoscopy Reveals Subclinical Arteritis and Periphlebitis After Intravitreal Brolucizumab Injection., PMID:39890100
Treatment of neovascular age-related macular degeneration: one year real-life results with intravitreal Brolucizumab., PMID:39886454
Comparative Pharmacokinetic Analysis of Aflibercept and Brolucizumab in Human Aqueous Humor Using Nano-Surface and Molecular-Orientation Limited Proteolysis., PMID:39859273
Intraocular inflammation after intravitreal injection of aflibercept 8 mg for treatment-refractory neovascular age-related macular degeneration: a case report., PMID:39849403
Efficacy of switching to brolucizumab for neovascular age-related macular degeneration refractory to faricimab., PMID:39847181
Brolucizumab for treating neovascular age-related macular degeneration: An alternative for treatment-refractory patients., PMID:39835444
Intraocular Inflammation Following Intravitreal Faricimab Injection in Neovascular Age-Related Macular Degeneration., PMID:39830553
Efficacy & safety of brolucizumab 6.0 mg versus 3.6 mg in diabetic macular edema., PMID:39806501
Drugs for age-related macular degeneration., PMID:39787576
Brolucizumab for the Treatment of Diabetic Macular Edema: An Optical Coherence Tomography-Based Analysis., PMID:39767218
"Triple and Plan" (TriPla) regimen for long lasting new generation intravitreal anti-VEGF., PMID:39763327
Brolucizumab and Platelet Activation and Reactivity., PMID:39760267