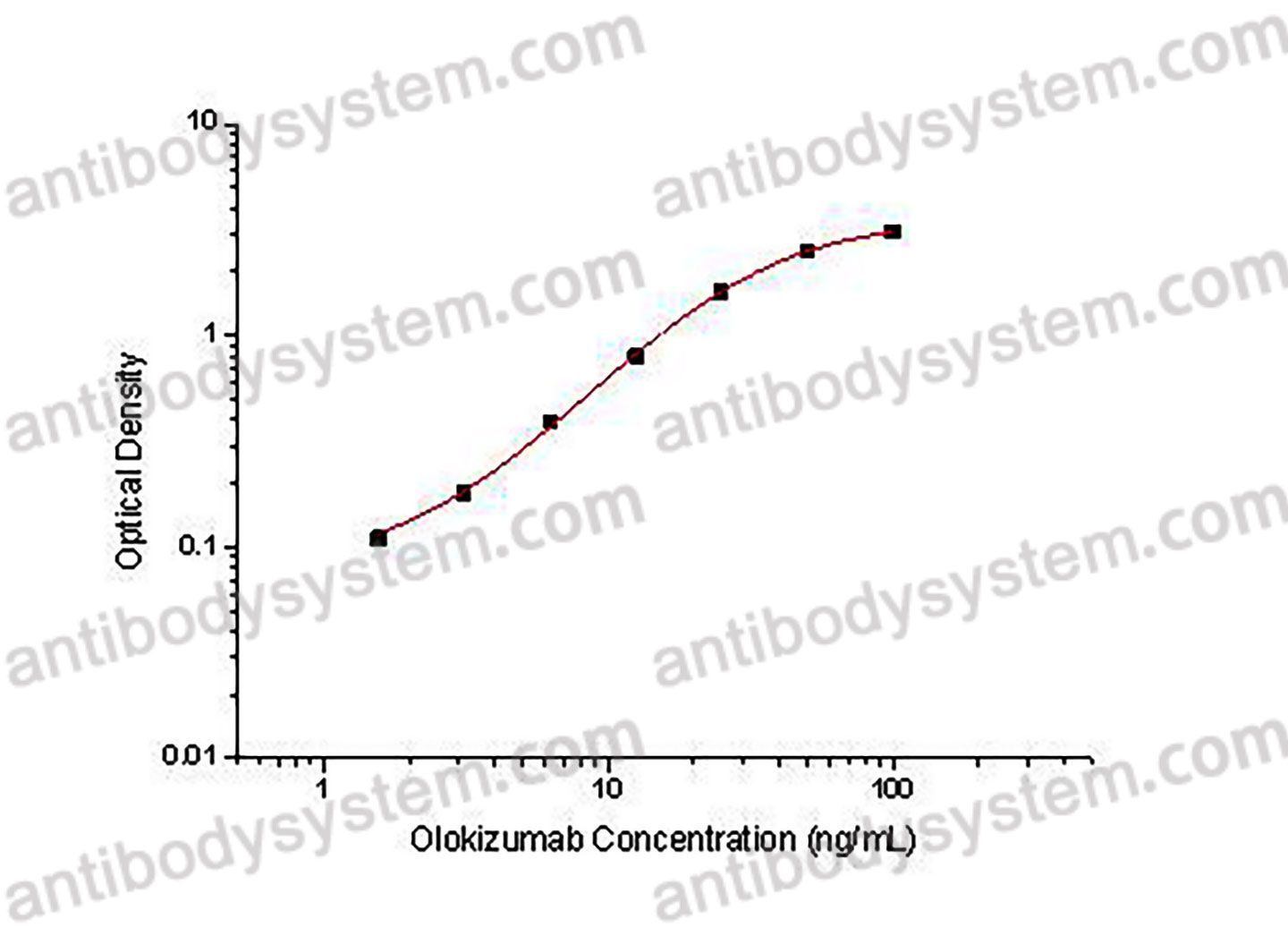
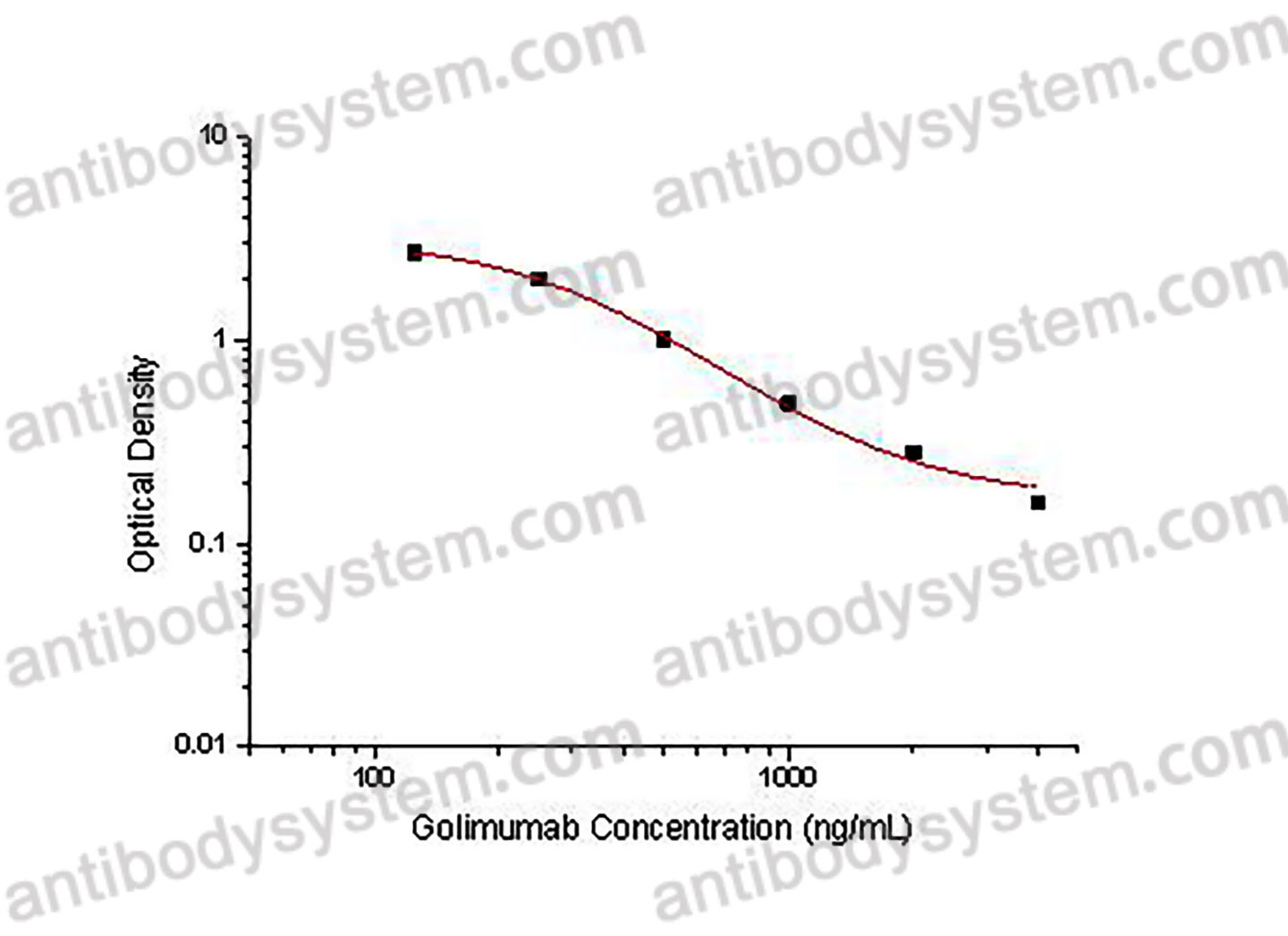
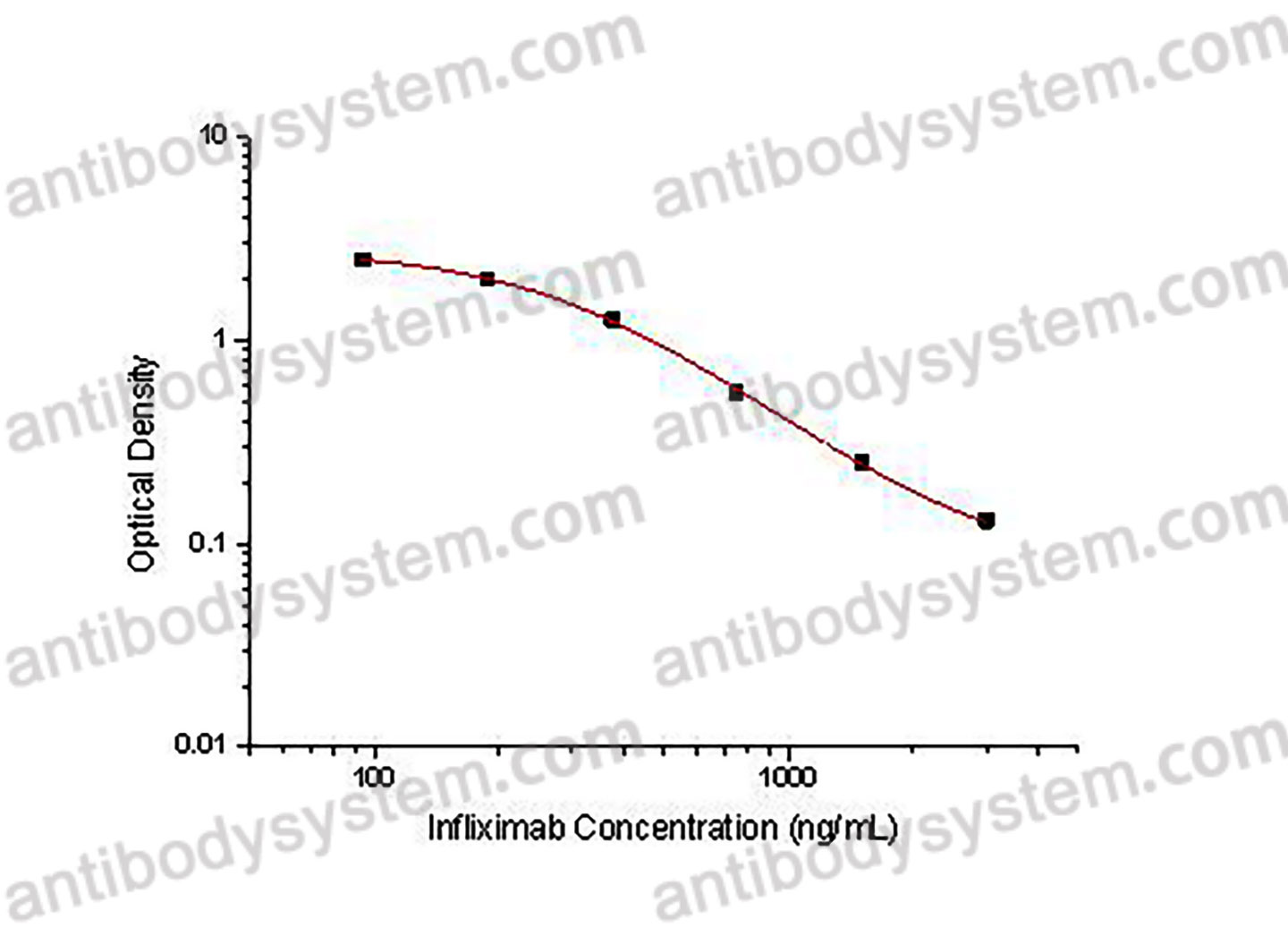
Catalog No.
KDD06801
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
0.31-5 μg/mL
Sensitivity
0.156 μg/ml
Precision
CV<20%
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
GS-5745, CAS: 1518996-49-0
Background
Andecaliximab (formerly GS-5745; Gilead Sciences, Inc.), a recombinant chimeric IgG4 monoclonal antibody (mAb), was engineered to remove T-cell epitopes to reduce immunogenicity risk. Andecaliximab selectively binds and inhibits matrix metalloproteinase-9 (MMP9) with minimal cross-reactivity to other matrix metalloproteinases, including the highly homologous matrix metalloproteinase-2 (MMP-2). Andecaliximab is under development for the treatment of cystic fibrosis, gastric cancer, pancreatic cancer, non-small cell lung cancer (NSCLC), rheumatoid arthritis (RA), Crohn's disease (CD), and ulcerative colitis (UC). In a recent phase 1 dose-escalation study in patients with UC, andecaliximab had good tolerability and was associated with a numerically greater percentage of clinical, endoscopic, and histological responses in patients relative to placebo over a 5-week treatment period. A phase 2/3 trial, evaluating the safety and efficacy of andecaliximab to induce and maintain clinical remission in patients with moderate to severe UC, was initiated. A planned interim futility analysis following an 8-week induction period in the first 150 patients resulted in the termination of the study due to lack of efficacy.
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Inside a Metastatic Fracture: Molecular Bases and New Potential Therapeutic Targets., PMID:40304052
Text Mining and Drug Discovery Analysis: A Comprehensive Approach to Investigate Diabetes-Induced Osteoporosis., PMID:38250601
Identification of key genes in colorectal cancer diagnosis by co-expression analysis weighted gene co-expression network analysis., PMID:36931200
Characterization of Active MMP9 in Chronic Inflammatory Diseases Using a Novel Anti-MMP9 Antibody., PMID:36810514
Evaluation of the matrix metalloproteinase 9 (MMP9) inhibitor Andecaliximab as an Anti-invasive therapeutic in Head and neck squamous cell carcinoma., PMID:35803110
A phase 1b study of andecaliximab in combination with S-1 plus platinum in Japanese patients with gastric adenocarcinoma., PMID:35773363
Safety and tolerability of andecaliximab as monotherapy and in combination with an anti-PD-1 antibody in Japanese patients with gastric or gastroesophageal junction adenocarcinoma: a phase 1b study., PMID:34992093
Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival., PMID:34893523
Matrix metalloproteinases in the pathogenesis of dengue viral disease: Involvement of immune system and newer therapeutic strategies., PMID:33634515
Phase III Study to Evaluate Efficacy and Safety of Andecaliximab With mFOLFOX6 as First-Line Treatment in Patients With Advanced Gastric or GEJ Adenocarcinoma (GAMMA-1)., PMID:33577358
Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis., PMID:33052708
Safety and Efficacy of Andecaliximab (GS-5745) Plus Gemcitabine and Nab-Paclitaxel in Patients with Advanced Pancreatic Adenocarcinoma: Results from a Phase I Study., PMID:32812320
Emerging Therapies in the Management of Advanced-Stage Gastric Cancer., PMID:30271341
Failure of MMP-9 Antagonists in IBD: Demonstrating the Importance of Molecular Biology and Well-Controlled Early Phase Studies., PMID:30010739
A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn's Disease., PMID:29846530
Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease., PMID:29767728
Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study., PMID:29691300
Phase 1b Study of the Safety, Pharmacokinetics, and Disease-related Outcomes of the Matrix Metalloproteinase-9 Inhibitor Andecaliximab in Patients With Rheumatoid Arthritis., PMID:29287749
Letter: anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 for ulcerative colitis., PMID:27593425
Randomised clinical trial: a phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis., PMID:27218676