Catalog No.
KDD00601
Description
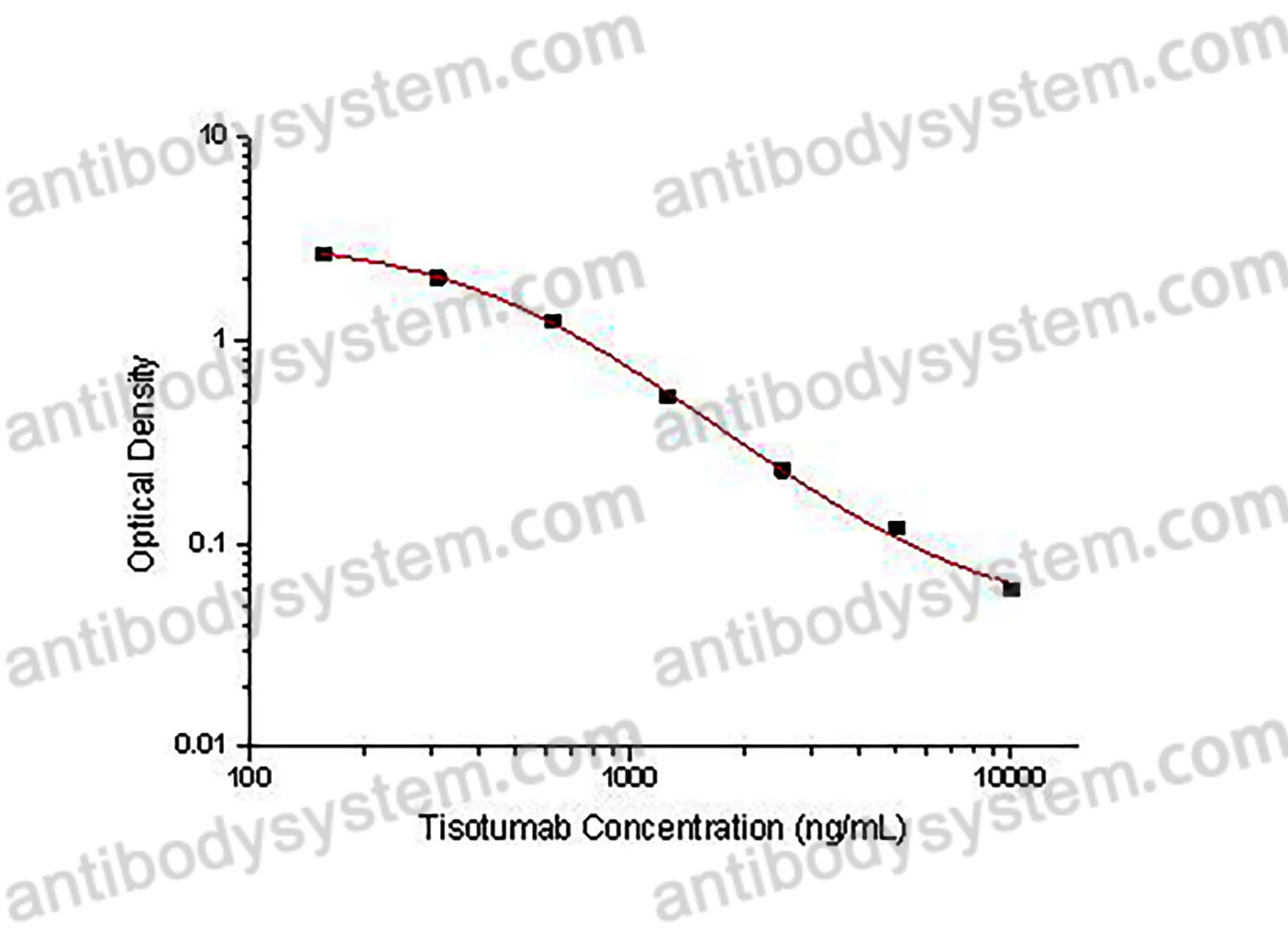
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD142 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tisotumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tisotumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tisotumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Tisotumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
159.18 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
3888.5
|
1175.5
|
271.1
|
4051.6
|
1179.5
|
249.5
|
|
Standard deviation
|
284.5
|
59.7
|
22.1
|
369.4
|
89.4
|
34.2
|
|
CV (%)
|
7.3
|
5.1
|
8.2
|
9.1
|
7.6
|
13.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
HuMax-TF-ADC, TF-011-MMAE, HuMax-TF, CAS: 1418628-81-5
Background
Tisotumab vedotin, also known as HuMax-TF, HuMax®-TF-ADC or TF-011-MMAE, is an antibody-drug conjugate (ADC) targeted to tissue factor (TF), a protein involved in tumor signaling and angiogenesis. Tisotumab vedotin includes an antibody targeting TF conjugated with monomethyl auristatin E (MMAE) via a cleavable maleimidocaproyl-valyl-citrullinyl-p-aminobenzyloxycarbonyl (mc-val-cit-PABC) type linker. Based on its high expression on many solid tumors (including ovarian, prostate, bladder, esophageal, endometrial and lung) and its rapid internalization, TF is considered a suitable target for antibody-drug conjugates. In pre-clinical trials tisotumab vedotin has shown strong ability to bind to TF and inhibit tumor growth. Genmab and Seattle Genetics are jointly developing tisotumab vedotin. In a Phase IIa study, preliminary data demonstrated a manageable safety profile and encouraging efficacy (ORR 37%) in relapsed, recurrent or metastatic cervical cancer.
The efficacy and safety of Tisotumab vedotin in the treatment of recurrent/metastatic cervical cancer: a systematic review and meta-analysis of single-arm studies., PMID:40458792
Rapidly progressive pneumonitis days after receiving tisotumab vedotin: a new antibody-drug conjugate., PMID:40234078
PMDA regulatory update on approval and revision of the precautions for use of anticancer drugs; approval of amivantamab plus lazertinib for non-small cell lung cancer, durvalumab for small cell lung cancer, tislelizumab for esophageal cancer, tisotumab vedotin for cervical cancer, ivosidenib for leukemia, and venetoclax for lymphoma in Japan., PMID:40214878
Clinical applications of antibody drug conjugates for gynecologic malignancies: Review of available medicines and emerging therapeutics., PMID:40139025
Second-line antibody-drug conjugates for the treatment of metastatic human papillomavirus-independent gastric-type adenocarcinoma of cervix: The Singapore experience., PMID:40115377
F3 Expression Drives Sensitivity to the Antibody-Drug Conjugate Tisotumab Vedotin in Glioblastoma., PMID:40075681
Practical clinical management of ocular adverse events related to Antibody-Drug Conjugates in gynaecological malignancies., PMID:39970828
Ocular surface disease related to tisotumab vedotin-tftv., PMID:39877468
Tissue factor targeted near-infrared photoimmunotherapy: a versatile therapeutic approach for malignancies., PMID:39751657
Ocular adverse events associated with antibody-drug conjugates: a comprehensive pharmacovigilance analysis., PMID:39742259
Antibody-Drug Conjugates: The Toxicities and Adverse Effects That Emergency Physicians Must Know., PMID:39641680
Antibody-Drug Conjugates: A Start of a New Era in Gynecological Cancers., PMID:39590153
Tisotumab vedotin extravasation injury in a patient with recurrent cervical cancer., PMID:39431057
Safety and efficacy of tisotumab vedotin with cervical cancers: A systematic review and meta-analysis., PMID:39428336
Real-World Large Sample Assessment of Drug-related Dry Eye Risk: Based on the FDA Adverse Event Reporting System Database., PMID:39343068
Antibody drug conjugates in recurrent or metastatic cervical cancer: a focus on tisotumab vedotin state of art., PMID:39323928
Anti-tissue factor antibody conjugated with monomethyl auristatin E or deruxtecan in pancreatic cancer models., PMID:39322584
Pharmacovigilance study of the association between peripheral neuropathy and antibody-drug conjugates using the FDA adverse event reporting system., PMID:39271716
Discrepancy in PD-L1 expression between primary and metastatic tumors in two patients with recurrent cervical cancer., PMID:39252760
[Antibody-Drug Conjugates in Breast Cancer and Gynecologic Cancer]., PMID:39191683
Accuracy and Completeness of Large Language Models About Antibody-Drug Conjugates and Associated Ocular Adverse Effects., PMID:39110155
Tisotumab vedotin effective in recurrent cervical cancer., PMID:39039198
Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer., PMID:38959480
Tisotumab vedotin (Tivdak) for cervical cancer., PMID:38905529
Ocular toxicities associated with antibody drug conjugates., PMID:38814581
Exploring tisotumab vedotin in recurrent cervical cancer: A case series including an HPV-independent gastric type adenocarcinoma., PMID:38523623
Cost effectiveness of immunotherapy combination therapies for endometrial cancer., PMID:38449799
Recent Therapeutic Advances in Gynecologic Oncology: A Review., PMID:38398161
Cost-effectiveness of tisotumab vedotin as a second- or third-line therapy for cervical cancer., PMID:38330381
Acute keratoconjunctivitis associated with tisotumab vedotin-tftv for metastatic cervical cancer., PMID:38230392
Antibody-Drug Conjugates in Gynecologic Cancers., PMID:38172449
Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system., PMID:38100054
A review of the state of cervical cancer: updates from prevention to recurrent disease., PMID:37873756
An Antibody-Drug Conjugate Directed to Tissue Factor Shows Preclinical Antitumor Activity in Head and Neck Cancer as a Single Agent and in Combination with Chemoradiotherapy., PMID:37828725
New Paradigms in the Treatment of Cervical Cancer., PMID:37826852
SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models., PMID:37793853
Therapeutic Potential of Tisotumab Vedotin in the Treatment of Recurrent or Metastatic Cervical Cancer: A Short Report on the Emerging Data., PMID:37790898
Uncovering therapeutic opportunities in the clinical development of antibody-drug conjugates., PMID:37740463
Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study., PMID:37651655
Sequential Targeted Therapy for Advanced, Metastatic, and Recurrent Cervical Cancer: A Cost-Effectiveness Analysis of the Patient Journey., PMID:37646470
Antibody-Drug Conjugates in Solid Tumor Oncology: An Effectiveness Payday with a Targeted Payload., PMID:37631374
Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence., PMID:37568702
The abscopal effect of immune-radiation therapy in recurrent and metastatic cervical cancer: a narrative review., PMID:37539054
Exposure-safety and exposure-efficacy analyses for tisotumab vedotin for patients with locally advanced or metastatic solid tumors., PMID:37496366
Tissue factor (coagulation factor III): a potential double-edge molecule to be targeted and re-targeted toward cancer., PMID:37280670
Antibody-Drug Conjugates in Gynecologic Cancer., PMID:37229642
The evolving landscape of antibody-drug conjugates in gynecologic cancers., PMID:37023499
Tisotumab Vedotin Safety and Tolerability in Clinical Practice: Managing Adverse Events., PMID:37009403