Catalog No.
KDC98501
Description
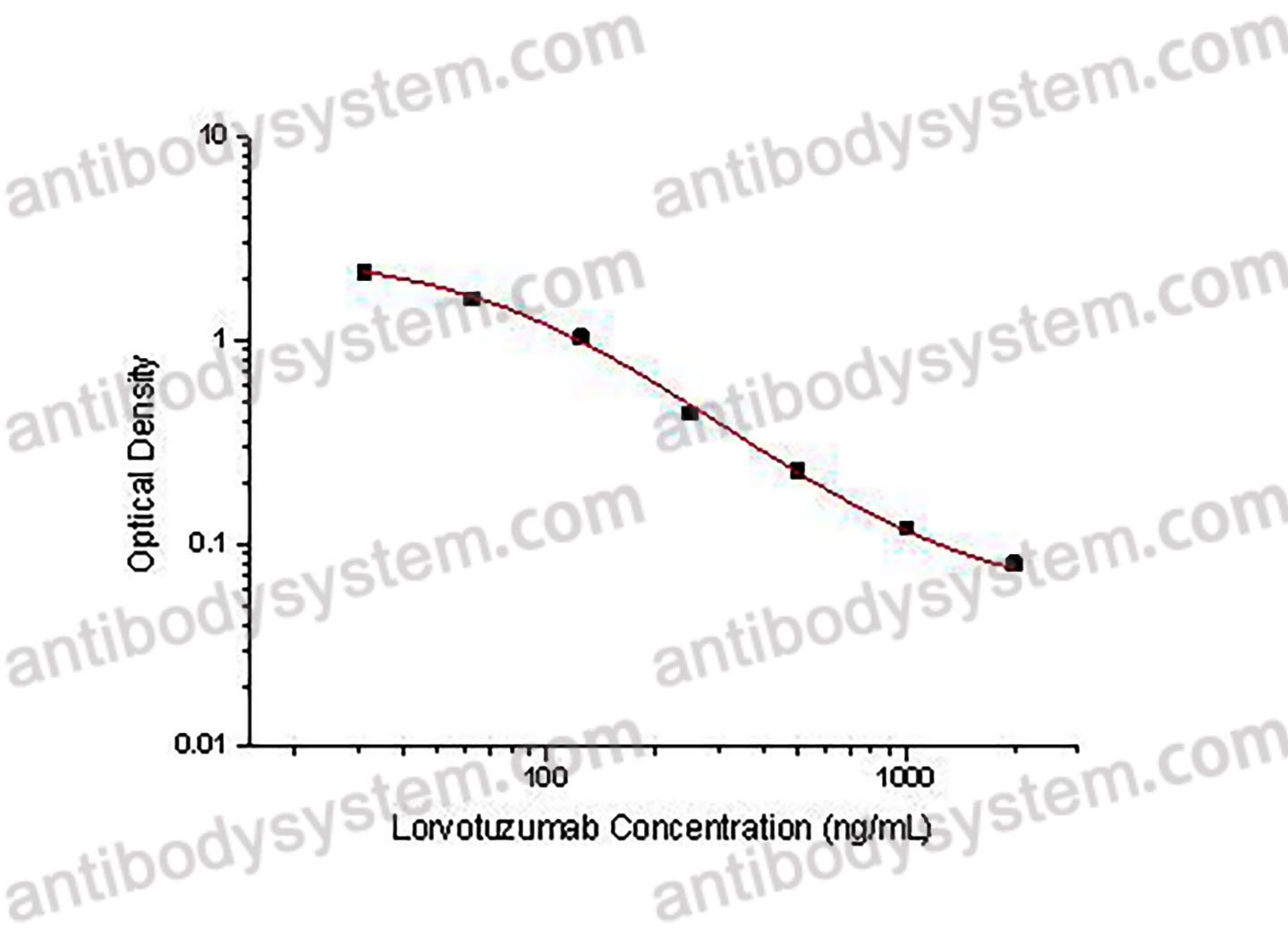
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD56 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Lorvotuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Lorvotuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Lorvotuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Lorvotuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
32.98 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
571.0 |
112.9 |
40.2 |
592.8 |
123.0 |
35.3 |
|
Standard deviation |
39.5 |
13.9 |
8.0 |
54.2 |
18.5 |
7.0 |
|
CV (%) |
6.9 |
12.3 |
20.0 |
9.1 |
15.0 |
19.9 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BB-10901, IMGN901, huN901-DM1, CAS: 1008106-64-6
Background
Lorvotuzumab mertansine (IMGN901, BB10901, huN901-DM1) is an antibody-drug conjugate (ADC) composed of the cytotoxic maytansinoid derivative, DM1, conjugated to the humanized N901 monoclonal antibody (lorvotuzumab, huN901), which binds CD56 with high affinity. DM1 conjugated to lorvotuzumab via a stable disulfide SPP linker (When DM1 is attached to an antibody with the SPP linker, it is mertansine; when it is attached with the thioether linker, SMCC, it is emtansine). It was developed by ImmunoGen, Inc. Lorvotuzumab mertansine is designed for the treatment of CD56 positive cancers (e.g. small-cell lung cancer, ovarian cancer). It has been granted orphan drug status for Merkel cell carcinoma and has reported encouraging Phase II results for small-cell lung cancer (SCLC). Once bound to CD56 on the surface of the target cell, the conjugate is internalized, the linker is cleaved, DM1 is released which in turn inhibits tubulin polymerization and results in cell death. Maytansine is a natural product, originally derived from the Ethiopian shrub Maytenus serrata. Maytansine inhibits tubulin polymerization, and is approximately 200-1,000 fold more cytotoxic than the Vinca alkaloids. Maytansine is clinically active but its narrow therapeutic window precluded further clinical development. Conjugation to an antibody and intracellular delivery could take advantage of the cytotoxic potency and expand the therapeutic window leading to greater tumor cell death with less overall toxicity. The maytansine derivative DM1 was developed specifically for use in ADCs.