Catalog No.
KDC90704
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
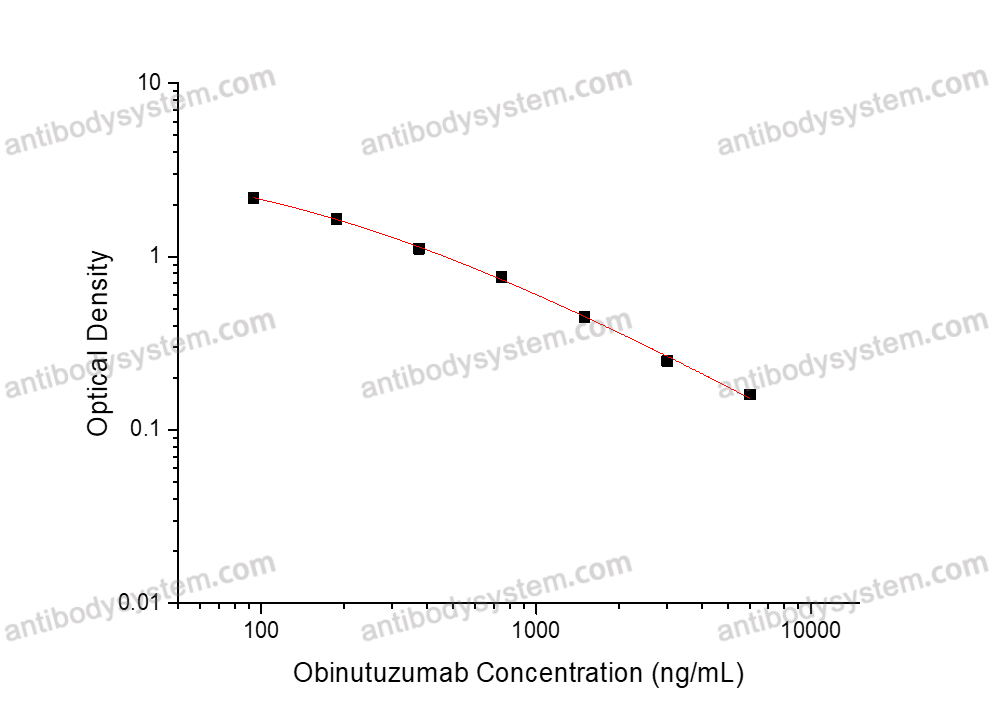
0.31-5 μg/mL
Sensitivity
0.156 μg/ml
Precision
CV<20%
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
CD20-TCB (2:1), RG-6026, CAS: 2229047-91-8
Background
Glofitamab (RG6026) is a novel T-cell-engaging, bispecific antibody that binds bivalently to CD20 on B cells, and monovalently to CD3 on T cells. In study NP30179 (NCT03075696), an ongoing Phase I/II dose-escalation and expansion study, glofitamab fixed-dosing (0.6-25mg) with obinutuzumab pre-treatment (Gpt) achieved high, durable CRs with manageable safety in pts with heavily pre-treated R/R NHL (Dickinson et al. EHA 2020). Step-up dosing (SUD) of glofitamab, in addition to Gpt, allowed dose-escalation up to the highest planned dose of 30mg to maximize efficacy, while mitigating cytokine release syndrome (CRS; [Hutchings et al. J Clin Oncol 2021]). We present updated duration of response (DoR) data from the glofitamab monotherapy fixed-dosing and SUD cohorts of study NP30179 in pts with R/R NHL.
Efficacy and safety of Glofitamab in patients with R/R DLBCL in real life setting- a retrospective study., PMID:40512187
From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies., PMID:40508128
QSP modeling of loncastuximab tesirine with T-cell-dependent bispecific antibodies guides dose-regimen strategy., PMID:40500294
CD20×CD3 Bispecific Antibodies in B-NHL: A Review of Translational Science, Pharmacokinetics, Pharmacodynamics, and Dose Strategy in Clinical Research., PMID:40471801
Pharmacological Insights on USFDA-Approved Novel Drug Therapies in the Year 2023., PMID:40464188
A Case Report of Sustained Cytokine Release Syndrome Due to Glofitamab and Literature Review., PMID:40322275
Recent development in bispecific antibody immunotherapy for hematological malignancies., PMID:40320222
Glofitamab with Polatuzumab Vedotin in Refractory Burkitt's Lymphoma., PMID:40305722
Cost-effectiveness analysis of glofitamab versus rituximab for relapsed or refractory diffuse large B-cell lymphoma patients in China., PMID:40293642
Successful treatment of primary refractory DLBCL/HGBL - MYC/BCL2 transformed from FL using glofitamab: a case report., PMID:40230856
Glofitamab results in cost savings versus epcoritamab in relapsed/refractory diffuse large B-cell lymphoma: a total cost of care analysis‡., PMID:40202060
Glofitamab in refractory or relapsed diffuse large B cell lymphoma after failing CAR-T cell therapy: a phase 2 LYSA study., PMID:40181090
Glofitamab induces deep and rapid response in relapsed primary central nervous system lymphoma., PMID:40174220
Budget impact of introducing glofitamab for treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy in the United States., PMID:40163049
Optimal Use of Bispecific Antibodies for the Treatment of Diffuse Large B-Cell Lymphoma in Canada., PMID:40136346
Enterocolitis associated with glofitamab-First report and clinicopathological findings in three cases., PMID:40108860
In vitro comparison of CD20xCD3 bispecific antibodies against diffuse large B-cell lymphoma (DLBCL) cell lines with different levels of expression of CD20., PMID:40032583
The Development and Application of Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma., PMID:39997328
Reply to: Glofitamab for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma., PMID:39951674
Glofitamab for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma., PMID:39951656
New bispecific antibodies in diffuse large B-cell lymphoma., PMID:39911111
The emergence of bispecific T-cell engagers in the treatment of follicular and large B-cell lymphomas., PMID:39820375
Salvage therapy for Burkitt lymphoma with glofitamab: a case report., PMID:39749407
Glofitamab in patients with HIV-associated B-cell lymphoma., PMID:39711495
Structural insight into CD20/CD3-bispecific antibodies by molecular modeling., PMID:39674067
Safety and Efficacy of Glofitamab for Relapsed/Refractory Large B-Cell Lymphoma in a Multinational Real-World Study., PMID:39661985
Bispecific antibodies for the treatment of hematologic malignancies: The magic is T-cell redirection., PMID:39617677
Benefit with glofitamab plus chemotherapy in transplant-ineligible R/R DLBCL., PMID:39609624
Dexamethasone is associated with reduced frequency and intensity of cytokine release syndrome compared with alternative corticosteroid regimens as premedication for glofitamab in patients with relapsed/refractory large B-cell lymphoma., PMID:39605203
Glofitamab plus gemcitabine and oxaliplatin (GemOx) versus rituximab-GemOx for relapsed or refractory diffuse large B-cell lymphoma (STARGLO): a global phase 3, randomised, open-label trial., PMID:39550172
Novel Bispecific T-Cell Engagers for the Treatment of Relapsed B Cell Non-Hodgkin Lymphomas: Current Knowledge and Treatment Considerations., PMID:39479221
The landscape of T-cell engagers for the treatment of follicular lymphoma., PMID:39398477
Glofitamab in Relapsed/Refractory Mantle Cell Lymphoma: Results From a Phase I/II Study., PMID:39365960
[Recent advances in the treatment of DLBCL]., PMID:39358300
Safety and Efficacy of Bispecific Antibodies in Adults with Large B-Cell Lymphomas: A Systematic Review of Clinical Trial Data., PMID:39273684
The Evolving Role of Bispecific Antibodies in Diffuse Large B-Cell Lymphoma., PMID:39063920
Tolerance of radiotherapy with concomitant glofitamab in diffuse large B cell lymphoma: a case report., PMID:38955824
Practice efficiency and total cost of care with bispecifics and CAR-T in relapsed/refractory diffuse large B-cell lymphoma: an institutional perspective., PMID:38913826
Glofitamab-Associated Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) Presenting as Serial Seizures and Responding Positively to Antiseizure Drugs and Anakinra: A Case Report., PMID:38910651
[Glofitamab in relapsed and/or refractory diffuse large B cell lymphoma after at least two lines of treatment]., PMID:38876895
SOHO State of the Art Updates and Next Questions | Current Evidence and Future Directions for Bispecific Antibodies in Large B-Cell Lymphoma., PMID:38871556
Relapse after glofitamab, a novel unmet medical need with high rates of CD20 loss., PMID:38813657
Bispecific antibodies in the treatment of relapsed/refractory large B-cell lymphoma., PMID:38809821
Allogeneic transplantation after immunotherapy for relapsed/refractory non-Hodgkin lymphoma: a comparison with a historical cohort., PMID:38775776
[T-cell recruiting immunotherapies in B-cell lymphoma - the future backbone for all therapy lines?]., PMID:38749439
Predictive model for the risk of cytokine release syndrome with glofitamab treatment for diffuse large B-cell lymphoma., PMID:38743882
Relapse after glofitamab has a poor prognosis and rates of CD20 loss are high., PMID:38720530
Disrupting B and T-cell collaboration in autoimmune disease: T-cell engagers versus CAR T-cell therapy?, PMID:38642912