Catalog No.
KDC82802
Description
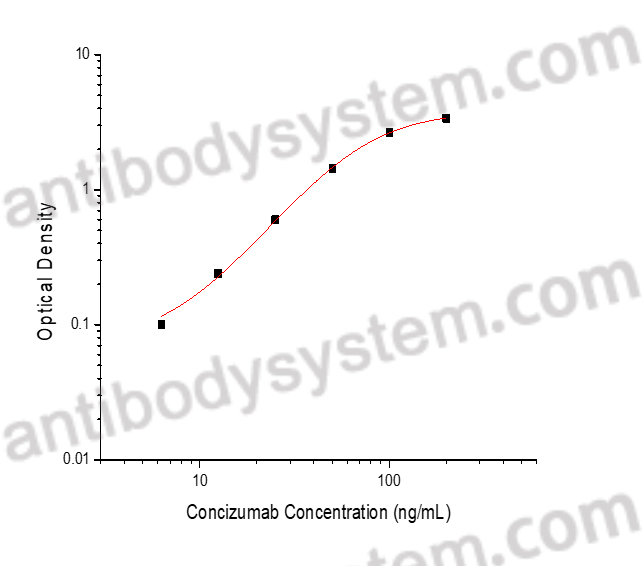
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Human TFPI has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Concizumab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Concizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Concizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
6.25 - 200 ng/mL
Sensitivity
3.58 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
80.4
|
24.8
|
11.8
|
76.1
|
24.3
|
12.2
|
|
Standard deviation
|
4.2
|
0.9
|
0.8
|
3.8
|
1.1
|
0.9
|
|
CV (%)
|
5.3
|
3.7
|
6.6
|
4.9
|
4.4
|
7.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
Anti-TFPI, NN7415, mab2021, CAS: 1312299-39-0
Background
Concizumab (mAb 2021) is a high-affinity, humanized, monoclonal IgG4 antibody (mAb) developed by Novo Nordisk A/S. It was directed against the Kunitz-2 domain of human tissue factor (TF) pathway inhibitor (TFPI), designed to target and selectively block the factor X (FX) a (FXa) binding site of TFPI. As a mAb, concizumab has the advantage of being administered subcutaneously, and exhibits good solubility and stability, allowing administration as a liquid formulation via a ready- and easy-to-use portable pen device. Phase 2 trials of concizumab are ongoing for Haemophilia A and B. On 12 October 2017, orphan designation (EU/3/17/1940) was granted by the European Commission to Novo Nordisk A/S, Denmark, for concizumab for the treatment of haemophilia B.
Anti-tissue factor pathway inhibitors for hemophilia: are these treatments the answer to overcoming current treatment limitations?, PMID:40515600
Evaluating the Safety and Efficacy of Concizumab in Hemophilia A/B Patients: A Systematic Review., PMID:40368339
Concizumab, a Non-Replacement Therapy for Persons with Hemophilia with Inhibitors., PMID:40363993
Case Report: Severe hemophilia B patient with inhibitor and anaphylaxis reaction to FIX, successfully managed with concizumab prophylaxis therapy., PMID:40356785
Concizumab (Alhemo) for hemophilia A and B with inhibitors., PMID:40324964
Revolutionizing Treatment Strategies through Inhibition of Tissue Factor Pathway Inhibitor: A Promising Therapeutic Approach for Hemophilia Management., PMID:40200623
Erratum: The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference [Corrigendum]., PMID:40171517
Concizumab prophylaxis in people with hemophilia A or B without inhibitors: patient-reported outcome results from the phase 3 explorer8 study., PMID:40166710
Advances in Development of Drug Treatment for Hemophilia with Inhibitors., PMID:39698264
Non-factor Therapies for Hemophilia: Achievements and Perspectives., PMID:39613145
Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach., PMID:39536817
Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): a prospective, multicentre, open-label, randomised, phase 3a trial., PMID:39521008
The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference., PMID:39161804
Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study., PMID:39099801
Disease and treatment burden of patients with haemophilia entering the explorer6 non-interventional study., PMID:39030946
Minimal interference of concizumab with standard clinical coagulation laboratory assays - An in vitro study., PMID:38924198
Targeting tissue factor pathway inhibitor with concizumab to improve hemostasis in patients with Glanzmann thrombasthenia: an in vitro study., PMID:38880178
Concizumab improves clot formation in hemophilia A under flow., PMID:38815755
Benefits and risks of non-factor therapies: Redefining haemophilia treatment goals in the era of new technologies., PMID:38481077
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B., PMID:38411279
[Angiology and hemostasis: what's new in 2023]., PMID:38231093
Antibodies to watch in 2024., PMID:38178784
Phase 3 Trial of Concizumab in Hemophilia with Inhibitors., PMID:37646676
Correction to: Concizumab: First Approval., PMID:37535220
Concizumab: First Approval., PMID:37341887
The Use of Bypassing Treatment Strategies in Hemophilia and Their Effect on Laboratory Testing., PMID:37146647
The Implication of New Developments in Hemophilia Treatment on Its Laboratory Evaluation., PMID:36381757
Non-factor therapies for bleeding disorders: A primer for the general haematologist., PMID:36051064
[Progress of Non-Factor Products in Hemophilia Treatment--Review]., PMID:35981403
The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V., PMID:35955419
Current and future therapies for haemophilia-Beyond factor replacement therapies., PMID:35869698
Managing invasive procedures in haemophilia patients with limited resources, extended half-life concentrates or non-replacement therapies in 2022., PMID:35521735
Concizumab as a Subcutaneous Prophylactic Treatment Option for Patients with Hemophilia A or B: A Review of the Evidence and Patient's Perspectives., PMID:35465188
Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors., PMID:35290453
Monitoring of new therapies for hemophilia., PMID:35088769
Thrombin generation for monitoring hemostatic therapy in hemophilia A: A narrative review., PMID:35034413
Thrombosis and novel hemophilia therapies: the fine line between clotting and bleeding., PMID:34581771
Advances in the management of haemophilia: emerging treatments and their mechanisms., PMID:34521404
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management., PMID:34026796
Understanding the evolution of coverage policies for prophylaxis treatments of hemophilia A without inhibitors: a payer Delphi panel., PMID:33843253
Thrombin generation potential in the presence of concizumab and rFVIIa, APCC, rFVIII, or rFIX: In vitro and ex vivo analyses., PMID:33819375
Post-authorization pharmacovigilance for hemophilia in Europe and the USA: Independence and transparency are keys., PMID:33810898
Concizumab: a novel anti-TFPI therapeutic for hemophilia., PMID:33570646
Light and shadows of the new therapies for haemophilia treatment in the COVID-19 era., PMID:32955428
The availability of new drugs for hemophilia treatment., PMID:32515633
Investigational drugs to treat hemophilia., PMID:32008381
TFPI blockade: removing coagulation's brakes., PMID:31778542
Paradigm shift for the treatment of hereditary haemophilia: Towards precision medicine., PMID:31676141
Concizumab promotes haemostasis via a tissue factor-factor VIIa-dependent mechanism supporting prophylactic treatment of haemophilia: Results from a rabbit haemophilia bleeding model., PMID:31609513