Catalog No.
KDC82401
Description
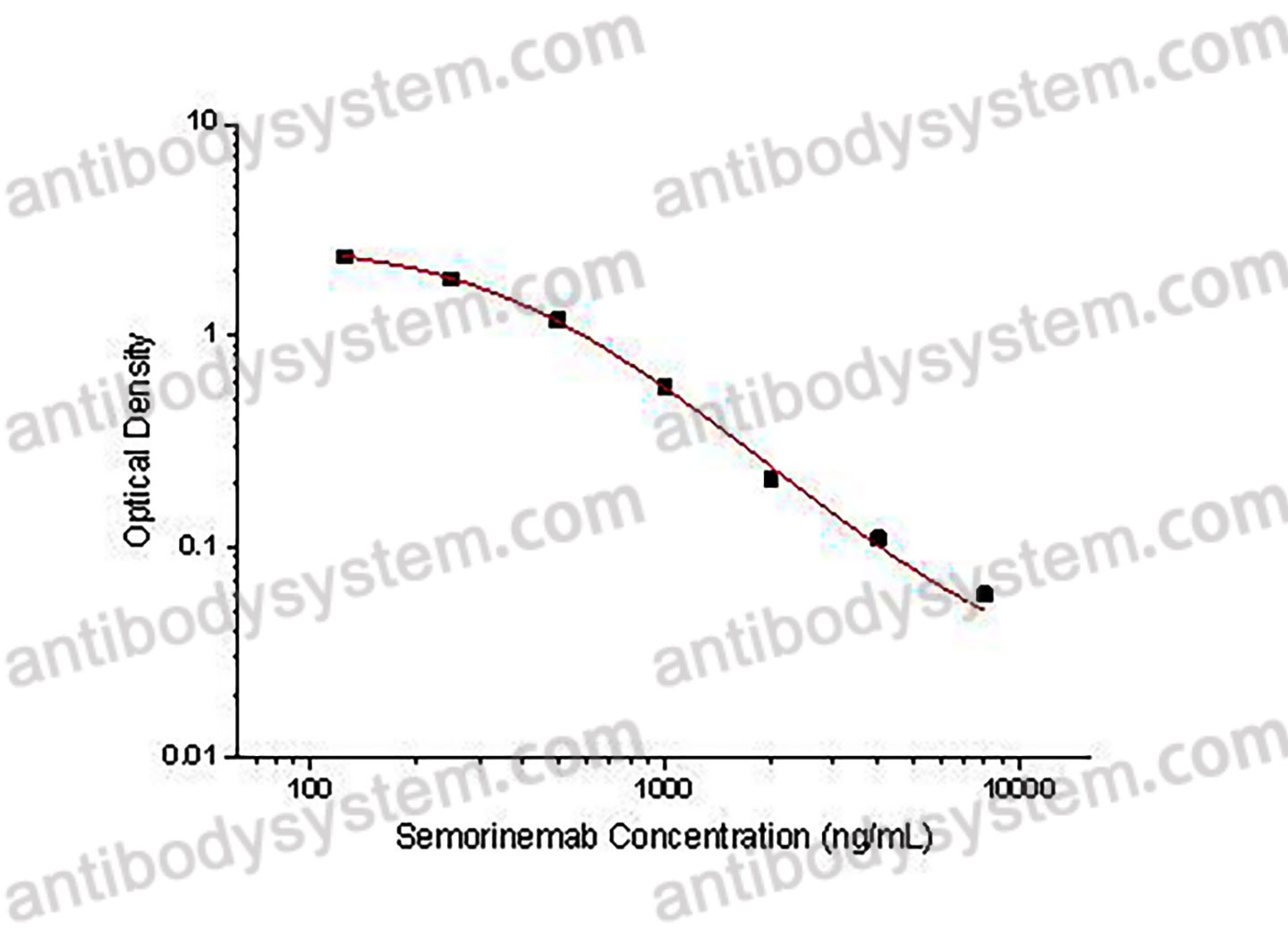
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human MAPT has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Semorinemab in the sample competitively binds to the pre-coated protein with biotin-labeled Semorinemab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Semorinemab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Semorinemab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 8,000 ng/mL
Sensitivity
21.46 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
3609.3
|
957.9
|
224.9
|
3439.5
|
950.8
|
199.3
|
|
Standard deviation
|
326.1
|
53.9
|
35.6
|
266.8
|
72.2
|
38.5
|
|
CV (%)
|
9.0
|
5.6
|
15.8
|
7.8
|
7.6
|
19.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
RO-7105705, CAS: 2159141-27-0
CSF proteomics of semorinemab Alzheimer's disease trials identifies cell-type specific signatures., PMID:40435316
The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer's disease based solely on biomarker evidence., PMID:40243238
Lecanemab for early Alzheimer's disease: Appropriate use recommendations from the French federation of memory clinics., PMID:40011173
Comparative the efficacy and safety of Gosuranemab, Semorinemab, Tilavonemab, and Zagotenemab in patients with Alzheimer's disease: a systematic review and network meta-analysis of randomized controlled trials., PMID:39945003
Phase 2A Proof-of-Concept Double-Blind, Randomized, Placebo-Controlled Trial of Nicotinamide in Early Alzheimer Disease., PMID:39671543
Pharmacodynamic effects of semorinemab on plasma and CSF biomarkers of Alzheimer's disease pathophysiology., PMID:39513754
2024 AA criteria for Alzheimer's disease diagnosis: Mainly anchored at Aβ not tau., PMID:39470319
CSF complement proteins are elevated in prodromal to moderate Alzheimer's disease patients and are not altered by the anti-tau antibody semorinemab., PMID:39369294
Semorinemab Pharmacokinetics and The Effect on Plasma Total Tau Pharmacodynamics in Clinical Studies., PMID:39350369
Scenarios for the long-term efficacy of amyloid-targeting therapies in the context of the natural history of Alzheimer's disease., PMID:39073291
A Review of Recent Advances in the Management of Alzheimer's Disease., PMID:38756263
What is Alzheimer's disease? An analysis of nosological perspectives from the 20th and 21st centuries., PMID:38618742
Evaluation of partial volume correction and analysis of longitudinal [18F]GTP1 tau PET imaging in Alzheimer's disease using linear mixed-effects models., PMID:38606196
Prognostic and Predictive Factors in Early Alzheimer's Disease: A Systematic Review., PMID:38405341
Posterior cortical atrophy: new insights into treatments and biomarkers for Alzheimer's disease., PMID:38267172
Analysis of clinical failure of anti-tau and anti-synuclein antibodies in neurodegeneration using a quantitative systems pharmacology model., PMID:37658103
Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet., PMID:37643887
Semorinemab in Mild-to-Moderate Alzheimer Disease: A Glimmer of Hope Though Cautions Remain., PMID:37643886
Passive tau-based immunotherapy for tauopathies., PMID:37620094
Clinical development of passive tau-based immunotherapeutics for treating primary and secondary tauopathies., PMID:37405389
Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer's disease., PMID:37389302
Cross-sectional and longitudinal assessments of function in prodromal-to-mild Alzheimer's disease: A comparison of the ADCS-ADL and A-IADL-Q scales., PMID:37325545
Dementia prevention in memory clinics: recommendations from the European task force for brain health services., PMID:36895446
The Use of Episodic Memory Tests for Screening in Clinical Trials for Early Alzheimer's Disease: A Comparison of the Free and Cued Selective Reminding Test (FCSRT) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)., PMID:36641609
[Therapeutic news in Alzheimer’s disease: soon a disease-modifying therapy?]., PMID:35929392
Anti-Tau Antibody Semorinemab Fails to Slow Alzheimer Disease., PMID:35916858
Safety and Efficacy of Semorinemab in Individuals With Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial., PMID:35696185
Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer's disease., PMID:33980574
Alzheimer's disease: Recent treatment strategies., PMID:32941929