Catalog No.
KDC43301
Description
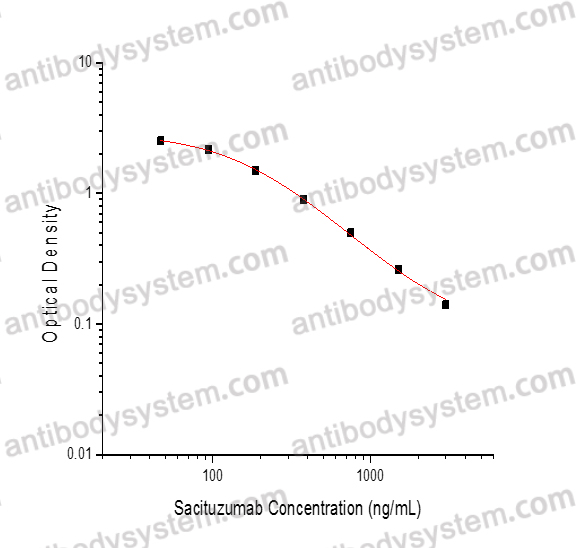
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human TACSTD2 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Sacituzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Sacituzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Sacituzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Sacituzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
46.88 - 3,000 ng/mL
Sensitivity
25.23 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1845.2
|
466.5
|
123.9
|
1806.2
|
422.6
|
100.3
|
|
Standard deviation
|
138.4
|
26.6
|
15.7
|
172.8
|
31.8
|
15.2
|
|
CV (%)
|
7.5
|
5.7
|
12.7
|
9.6
|
7.5
|
15.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
IMMU-132, hRS7-SN-38, hRS7-SN-38-ADC, hRS7-[CL-SN-38], , CAS: 1796566-95-4
Background
Sacituzumab govitecan (sacituzumab govitecan-hziy; Trodelvy™) is a Trop-2-directed antibody conjugated to a topoisomerase I inhibitor (SN-38) that is being developed by Immunomedics for the treatment of solid tumours, including breast cancer. In April 2020, sacituzumab govitecan received accelerated approval in the USA for the treatment of adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for metastatic disease. Sacituzumab govitecan is undergoing phase III development for breast cancer in the USA and EU, and phase II development for urothelial cancer. It is also being explored for brain metastases, glioblastoma, endometrial cancer and prostate cancer.
Advances in targeted therapy for triple-negative breast cancer: a review of key antigens and recent advances., PMID:40515614
The antibody-drug conjugate sacituzumab govitecan (IMMU-132) represents a potential novel therapeutic strategy in cholangiocarcinoma., PMID:40509933
A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician's Choice in Previously Treated HR+/HER- mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials., PMID:40507364
Advancing Antibody-Drug Conjugates: Precision Oncology Approaches for Breast and Pancreatic Cancers., PMID:40507272
Efficacy and Safety of TROP-2-Targeting Antibody-Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data., PMID:40507234
Comparative pharmacological analysis of fam-trastuzumab deruxtecan-nxki and sacituzumab govitecan-hziy: Two recently developed chemotherapies in the crucial battle against breast cancer., PMID:40502326
Synergistic strategies: ADC-PARP inhibitor combinations in triple-negative breast cancer therapy., PMID:40494034
Results of a phase 1/2 study of sacituzumab tirumotecan in patients with unresectable locally advanced or metastatic solid tumors refractory to standard therapies., PMID:40481574
Effectiveness of sacituzumab govitecan and management of neutropenia in patients with metastatic triple-negative breast cancer treated in real-world settings in the United States., PMID:40479864
Real-world clinical outcomes of sacituzumab govitecan after prior exposure to enfortumab vedotin in patients with metastatic urothelial carcinoma., PMID:40479863
Sacituzumab tirumotecan versus docetaxel for previously treated EGFR-mutated advanced non-small cell lung cancer: multicentre, open label, randomised controlled trial., PMID:40473437
The association of high body mass index with the safety and efficacy of sacituzumab govitecan in patients with metastatic triple-negative breast cancer from the ASCENT study., PMID:40460679
Mechanisms of resistance to antibody-drug conjugates in cancers., PMID:40460510
New Treatment Approaches for Triple-Negative Breast Cancer., PMID:40460322
Evaluation of sacituzumab govitecan for advanced/metastatic non-small cell lung cancer., PMID:40458070
Avelumab plus sacituzumab govitecan versus avelumab monotherapy as first-line maintenance treatment in patients with advanced urothelial carcinoma: JAVELIN Bladder Medley interim analysis., PMID:40456670
Sequential Antibody-Drug Conjugate Therapy in Patients With Metastatic Breast Cancer Treated With Sacituzumab Govitecan and Trastuzumab Deruxtecan., PMID:40440568
Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization., PMID:40427091
ADCs and TCE in SCLC Therapy: The Beginning of a New Era?, PMID:40422520
Cost and Cost-Effectiveness of Treating Human Epidermal Growth Factor Receptor 2-Low Metastatic Breast Cancer., PMID:40397834
A Multicenter Real-Life Evaluation of Safety and Effectiveness of the Antibody-Drug Conjugate Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer., PMID:40387297
Update Breast Cancer 2024 Part 3 - Patients with Advanced Stage Breast Cancer., PMID:40386501
Progress of antibody-drug conjugates in the treatment of locally advanced or metastatic urothelial carcinoma: opportunities and challenges., PMID:40377724
Plain language summary of the EVOKE-01 study of sacituzumab govitecan vs docetaxel in patients with non-small cell lung cancer., PMID:40371894
[Translated article] Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review., PMID:40368667
Decoding Clinical Trials in Metastatic Breast Cancer: Practical Insights for Optimal Therapy Sequencing., PMID:40367401
Delphi consensus on the management of adverse events in patients with metastatic triple-negative breast cancer treated with sacituzumab govitecan., PMID:40366333
The Clinical Outcomes and Safety of Sacituzumab Govitecan in Heavily Pretreated Metastatic Triple-Negative and HR+/HER2- Breast Cancer: A Multicenter Observational Study from Turkey., PMID:40361516
TROP2 expression and therapeutic targeting in uterine carcinosarcoma., PMID:40344963
Navigating antibody‒drug conjugates (ADCs): from metastatic to early breast cancer treatment strategies., PMID:40304863
MMP1-induced NF-κB activation promotes epithelial-mesenchymal transition and sacituzumab govitecan resistance in hormone receptor-positive breast cancer., PMID:40287412
Sacituzumab tirumotecan improves OS in mTNBC., PMID:40269173
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Antibody-Based Therapeutics in Small Cell Lung Cancer: A Narrative Review., PMID:40260055
Evaluation of Nectin-4 and Trop-2: Implications for Patient Outcomes and Therapy in Penile Cancer., PMID:40252843
Targeting Lung Cancer with Precision: The ADC Therapeutic Revolution., PMID:40238068
Multicenter retrospective cohort study of the sequential use of the antibody-drug conjugates (ADCs) trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) in patients with HER2-low metastatic breast cancer (MBC)., PMID:40234477
Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial., PMID:40217078
Sacituzumab tirumotecan in advanced non-small-cell lung cancer with or without EGFR mutations: phase 1/2 and phase 2 trials., PMID:40210967
Antibody-drug conjugates in breast cancer treatment: resistance mechanisms and the role of therapeutic sequencing., PMID:40201309
Trop2-expression in primary vulvar squamous cell carcinoma., PMID:40184712
Safety and Tolerability of Concurrent Radiotherapy and Sacituzumab Govitecan in Metastatic Breast Cancer., PMID:40178882
Advances in adoptive cell therapies in small cell lung cancer., PMID:40160238
Toxicities and management strategies of emerging antibody-drug conjugates in breast cancer., PMID:40151551
Direct Preparation of Site-Specific Antibody-Drug Conjugates with Unpurified Antibodies in Culture Medium., PMID:40151029
Camptothein-Based Anti-Cancer Therapies and Strategies to Improve Their Therapeutic Index., PMID:40149365
Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review., PMID:40140308
Q-TWiST Analysis of Sacituzumab Govitecan vs. Chemotherapy in Previously Treated Patients with HR+/HER2- Metastatic Breast Cancer., PMID:40136373
Correction to: Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial., PMID:40132065
Toxicities Associated with Sacituzumab Govitecan: Data from Clinical Trials and a Real-World Pharmacovigilance Database., PMID:40131630