Catalog No.
KDC29905
Description
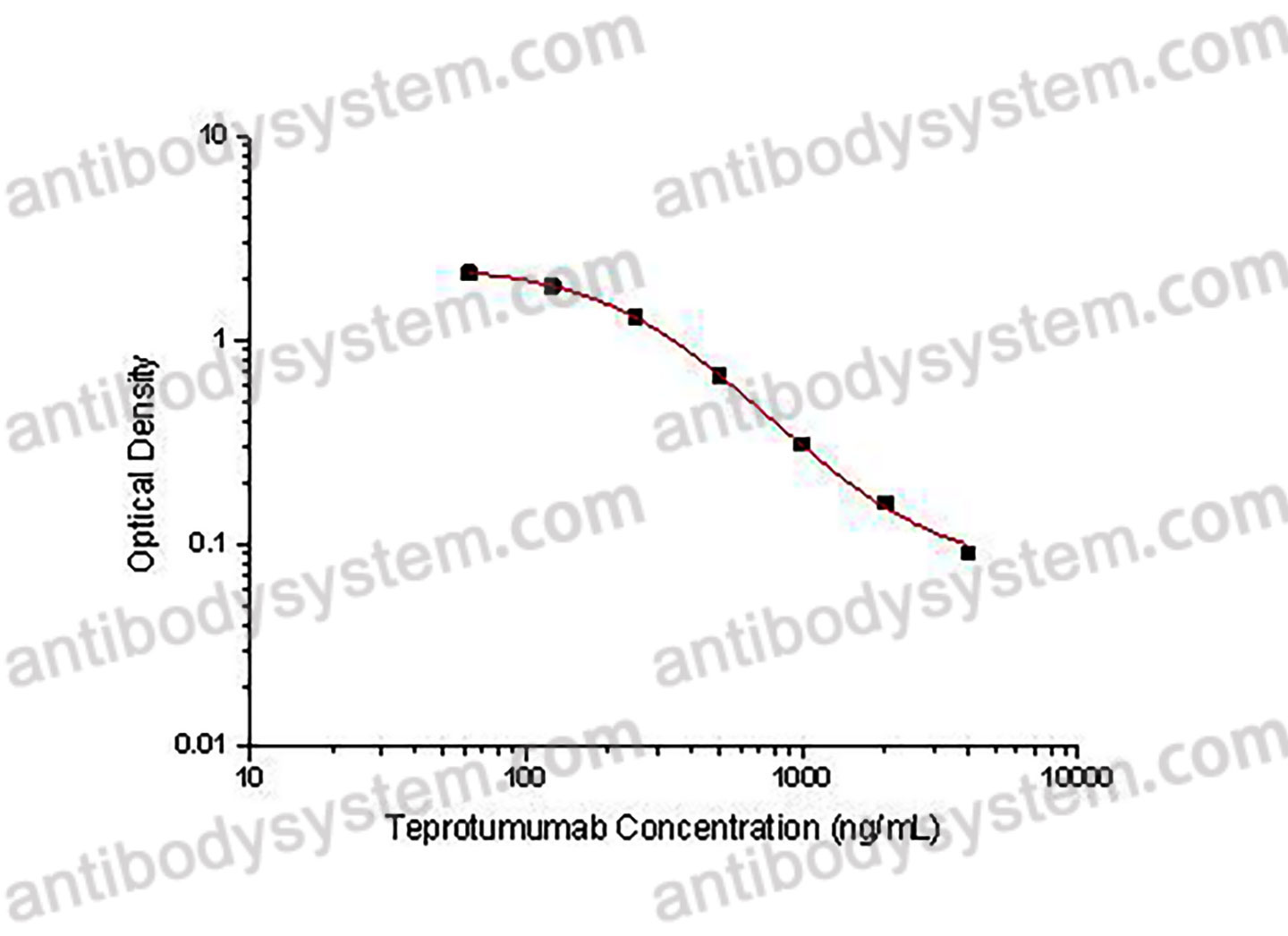
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IGF1R has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Teprotumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Teprotumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Teprotumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Teprotumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
45.51 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess
intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess
inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1779.8
|
521.3
|
147.2
|
1819.5
|
464.7
|
116.4
|
|
Standard deviation
|
51.5
|
9.3
|
12.3
|
99.3
|
29.4
|
14.6
|
|
CV (%)
|
2.9
|
1.8
|
8.4
|
5.5
|
6.3
|
12.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20℃, the rest reagents should be store at 4℃.
Alternative Names
RO4858696-000, CAS: 1036734-93-6
Background
Teprotumumab is a human IgG1 monoclonal antibody directed against the insulin-like growth factor 1 receptor (IGF1R) that is used in the therapy of Graves ophthalmopathy (thyroid eye disease). Graves disease is an autoimmune disease associated with autoantibodies, hyperthyroidism and a distinctive form of eye disease marked by ocular inflammation and proptosis causing eye pain, dryness, redness, and swelling which if severe can cause diplopia (double-vision) and loss of vision. Graves ophthalmopathy is associated with autoantibodies to thyrotropin and hypertrophy of ocular fibroblasts which overexpress the IGF1 receptor. Teprotumumab causes inhibition of IGF1 binding to its receptor and preventing cell activation. IGF1 is a potent modulator of cell growth and inhibitor of programmed cell death, acting via the PI3K-AKT-mTOR pathway. The inhibition of IGF1 signaling decreases fibroblast proliferation and hypertrophy.
Teprotumumab in the management of thyroid eye disease mechanistic insights and adverse reactions: a comprehensive review., PMID:40496563
Persistent Palmar Rash Associated with Teprotumumab Treatment., PMID:40488249
Regional Differences in the Management of Thyroid Eye Disease: Results from an International Clinical Practice Survey of Endocrinologists., PMID:40432588
Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves' orbitopathy: a systematic review and meta-analysis., PMID:40404973
Long-term Cardiovascular, Renal, and Safety Outcomes of Teprotumumab versus Systemic Glucocorticoids in Thyroid Eye Disease: A Target Trial Emulation., PMID:40398692
Thyroid eye disease in the biologic era: a 40-year paradigm shift in nonsurgical therapeutic strategies., PMID:40397653
Teprotumumab Use in Thyroid Eye Disease: Clinical Outcomes in the United Arab Emirates- a First Regional Case Series., PMID:40396469
Time to improvement following teprotumumab treatment of thyroid eye disease: real world experience., PMID:40366381
LSD1 induces H3 K9 demethylation to promote adipogenesis in thyroid-associated ophthalmopathy., PMID:40340927
Thyroid eye disease (Graves' orbitopathy): clinical presentation, epidemiology, pathogenesis, and management., PMID:40324443
Thyroid-related orbitopathy: clinical overview, novel medical treatments and the role of orbital surgery., PMID:40317422
Statins and Thyroid Eye Disease: A Propensity Score-Matched Retrospective Cohort Analysis., PMID:40309510
Efficacy and safety of Tocilizumab for thyroid eye disease: a systemic review and Meta-analysis., PMID:40304985
Teprotumumab Improves Quality of Life in Thyroid Eye Disease: Meta-analysis and Matching-adjusted Indirect Comparison., PMID:40303547
Orbital Decompression in the Biologic Era: Is There Still a Need for Surgery?, PMID:40299895
The role of IL-6 in thyroid eye disease: an update on emerging treatments., PMID:40297767
Drug-induced hearing loss: a real-world pharmacovigilance study using the FDA adverse event reporting system database., PMID:40188564
Targeted immunotherapies for Graves' thyroidal & orbital diseases., PMID:40145088
Multiple pseudoprogressions during ongoing immunotherapy-based treatment of advanced gastric neuroendocrine carcinoma: A case report and review of literature., PMID:40092963
Spectral-domain optical coherence tomography imaging findings in patients receiving teprotumumab for thyroid eye disease., PMID:40083365
Nationwide orbital decompression volume, surgical approach, and subspecialty distribution patterns within the center for medicare and medicaid services population in the era of teprotumumab., PMID:40079573
Efficacy and Safety of Teprotumumab in Thyroid Eye Disease: A Systematic Review and Meta-Analysis., PMID:39952471
Otologic Symptoms Following Teprotumumab Administration in Patients with Thyroid Eye Disease., PMID:39951668
Thyroid dermopathy and thyroid eye disease associated with hypofunctional autoimmune thyroid disease with high TSI/anti-TSHR antibodies improved with teprotumumab., PMID:39950657
Controversies Surrounding IGF-I Receptor Involvement in Thyroid-Associated Ophthalmopathy., PMID:39909461
A randomised, double-masked, placebo-controlled trial evaluating the efficacy and safety of teprotumumab for active thyroid eye disease in Japanese patients., PMID:39896230
Novel perspectives on the pharmacological treatment of thyroid-associated ophthalmopathy., PMID:39872310
Re: Ugradar et al.: The rate of re-treatment in patients treated with teprotumumab: a multicenter study of 119 patients with 1 year of follow-up (Ophthalmology. 2025;132:92-97)., PMID:39818625
Observational Characterization of the Retreatment Course of Patients With Thyroid Eye Disease., PMID:39780310
Reply Re: "Teprotumumab for the Treatment of Recalcitrant Thyroid Eye Disease"., PMID:39752221
Re: "Teprotumumab for the Treatment of Recalcitrant Thyroid Eye Disease"., PMID:39752220
Linsitinib inhibits proliferation and induces apoptosis of both IGF-1R and TSH-R expressing cells., PMID:39723207
Clinical and Radiologic Predictors of Response to Teprotumumab: A 3D Volumetric Analysis of 35 Patients., PMID:39714282
Efficacy, Safety, and Recurrence in Older Thyroid Eye Disease Patients Undergoing Teprotumumab Treatment., PMID:39704303
Epidemiology and Management of Moderate to Severe Thyroid Eye Disease in the United States: Analysis of a Healthcare Claims Database., PMID:39690929
Comprehensive Comparisons of Different Treatments for Active Graves Orbitopathy: A Systematic Review and Bayesian Model-Based Network Meta-Analysis., PMID:39680569
Risk of audiologic side effects with teprotumumab treatment for thyroid eye disease: propensity matched analysis., PMID:39668181
Surgical Timing for Patients With Thyroid Eye Disease Treated With Teprotumumab: A Collaborative Multicenter Study., PMID:39656059
Teprotumumab versus intravenous methylprednisolone in thyroid eye disease: A systematic review., PMID:39651498
Advances of IGF-1R inhibitors in Graves' ophthalmopathy., PMID:39578269
Teprotumumab's Impact on Proptosis in Long-duration Thyroid Eye Disease: A Systematic Review and Meta-analysis., PMID:39526058
Recent advances in neuro-ophthalmology., PMID:39462921
Teprotumumab for the Treatment of Thyroid Eye Disease: Why Should We Keep Our Eyes "Wide Open"?-A Clinical and Pharmacovigilance Point of View., PMID:39452535
[Survey of the management of moderate to severe active Graves' orbitopathy at 28 metropolitan centers of excellence in France]., PMID:39368261
The Impact of Monoclonal Antibody Usage on Hearing Outcomes: A Systematic Review., PMID:39268884
Reply Re: "Proptosis Regression After Teprotumumab Treatment for Thyroid Eye Disease"., PMID:39240200
Re: "Proptosis Regression After Teprotumumab Treatment for Thyroid Eye Disease"., PMID:39240199
Effect of Cannabis Usage on Thyroid Eye Disease., PMID:39197177
Patterns of Teprotumumab-Induced Hearing Dysfunction: A Systematic Review., PMID:39194388
Effects of Teprotumumab and Role of Human Leukocyte Antigens Markers in Patients With Thyroid Eye Disease., PMID:39187158