Catalog No.
KDC29903
Description
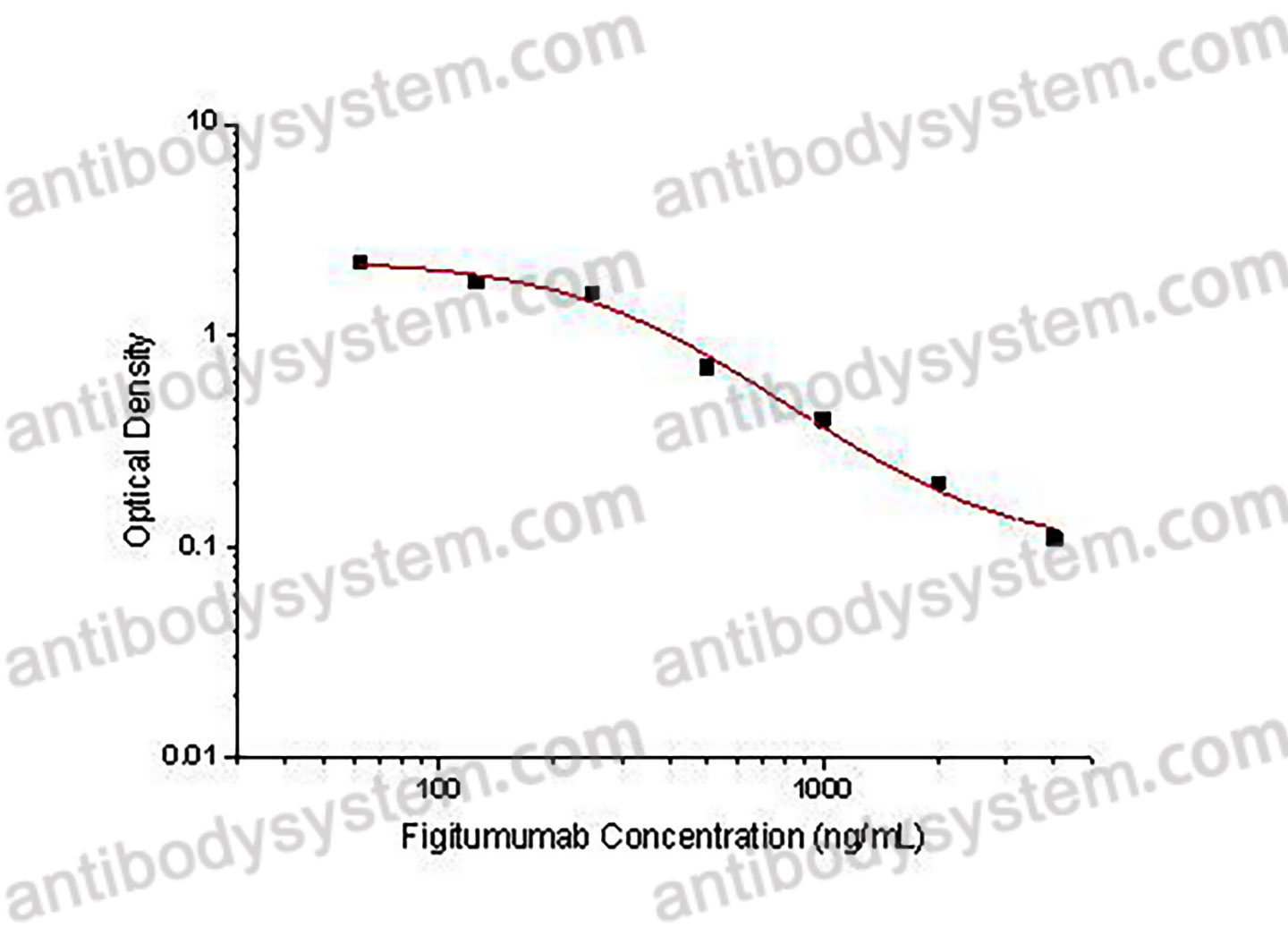
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD221 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Figitumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Figitumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Figitumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Figitumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
10.24 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2171.2
|
444.5
|
113.9
|
2165.2
|
487.5
|
132.8
|
|
Standard deviation
|
144.2
|
39.5
|
16.4
|
218.1
|
41.0
|
18.7
|
|
CV (%)
|
6.6
|
8.9
|
14.4
|
10.1
|
8.4
|
14.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CP-751871, CAS: 943453-46-1
Background
Figitumumab is a fully humanized IgG2 mAb against IGF-1R. Its recommended dose is 20 mg/kg repeated weekly. In phase I trials treatment-related toxicities were generally mild. The most common adverse events reported were hyperglycemia, anorexia, nausea, elevation of liver function tests, diarrhea, hyperuracemia and fatigue. Figitumumab had shown significant activity against non-small-cell lung cancer (NSCLC) and it was planned to be evaluated in combination with chemotherapy in a randomized phase III trial in patients with metastatic NSCLC. However, the study was early discontinued on December 2009 because an independent monitoring committee concluded that the combination of figitumumab plus chemotherapy would be unlikely to meet the primary endpoint of improving overall survival compared to chemotherapy alone. Additionally, there were also some concerns that hyperglycemia could be a potential contributor of increased patients’ morbidity. In regard to breast cancer, figitumumab was planned to be tested in phase I trials as neo-adjuvant treatment, but the trial was withdrawn prior initiation, although there are preclinical data showing a additive and/or synergistic effect of figitumumab with chemotherapy in basal breast cancer subtype.
Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors., PMID:38136399
The Immunotherapy Landscape in Adrenocortical Cancer., PMID:34071333
A Review of Monoclonal Antibody-Based Treatments in Non-small Cell Lung Cancer., PMID:33725344
Osteosarcoma Pathogenesis Leads the Way to New Target Treatments., PMID:33467481
Advances in antibody therapeutics targeting small-cell lung cancer., PMID:29790694
Emerging immune targets for the treatment of multiple myeloma., PMID:29421983
Enhanced response of melanoma cells to MEK inhibitors following unbiased IGF-1R down-regulation., PMID:29137261
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy., PMID:28427155
Co-targeting the HER and IGF/insulin receptor axis in breast cancer, with triple targeting with endocrine therapy for hormone-sensitive disease., PMID:28236033
IGFBP, a novel target of lung cancer?, PMID:28104361
Targeted therapies for the treatment of non-small-cell lung cancer: Monoclonal antibodies and biological inhibitors., PMID:27831000
[Clinical trials and licensing of monoclonal antibodies and biological medicines for cancer treatment in Brazil]., PMID:27754528
A Phase I Clinical Trial and Independent Patient-Derived Xenograft Study of Combined Targeted Treatment with Dacomitinib and Figitumumab in Advanced Solid Tumors., PMID:27733479
IGF1R Derived PI3K/AKT Signaling Maintains Growth in a Subset of Human T-Cell Acute Lymphoblastic Leukemias., PMID:27532210
Metformin Enhances the Therapy Effects of Anti-IGF-1R mAb Figitumumab to NSCLC., PMID:27488947
Importance of the type I insulin-like growth factor receptor in HER2, FGFR2 and MET-unamplified gastric cancer with and without Ras pathway activation., PMID:27437872
Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies., PMID:27377311
Insulin-like Growth Factor Receptor Inhibition as Maintenance Therapy for Metastatic Ewing Sarcoma., PMID:27322713
Emerging antibodies for the treatment of multiple myeloma., PMID:27195659
Insulin-like growth factors are essential to prevent anoikis in oestrogen-responsive breast cancer cells: importance of the type I IGF receptor and PI3-kinase/Akt pathway., PMID:26801096
Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade., PMID:26429980
Minireview: Were the IGF Signaling Inhibitors All Bad?, PMID:26366975
Combinational Therapy Enhances the Effects of Anti-IGF-1R mAb Figitumumab to Target Small Cell Lung Cancer., PMID:26287334
In vitro and in vivo studies of the combination of IGF1R inhibitor figitumumab (CP-751,871) with HER2 inhibitors trastuzumab and neratinib., PMID:26195122
The lesson learned from figitumumab clinical program and the hope for better results in squamous lung cancer., PMID:25806342
The failure of figitumumab: the danger of taking shortcuts in drug development., PMID:25524479
Randomized, phase III trial of figitumumab in combination with erlotinib versus erlotinib alone in patients with nonadenocarcinoma nonsmall-cell lung cancer., PMID:25395283
Antitumor effects and molecular mechanisms of figitumumab, a humanized monoclonal antibody to IGF-1 receptor, in esophageal carcinoma., PMID:25358597
Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer., PMID:24888810
Targeting receptor tyrosine kinases in gastric cancer., PMID:24782606
The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells., PMID:24743973
Emerging therapeutic targets for synovial sarcoma., PMID:24661286
Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer., PMID:24536060
Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: a nonrandomized, open-label, phase II trial., PMID:24488322
Metformin as an adjuvant drug against pediatric sarcomas: hypoxia limits therapeutic effects of the drug., PMID:24391834
Molecular predictors of sensitivity to the insulin-like growth factor 1 receptor inhibitor Figitumumab (CP-751,871)., PMID:24107449
The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy., PMID:24033707
Targeting non-small cell lung cancer cells by dual inhibition of the insulin receptor and the insulin-like growth factor-1 receptor., PMID:23826179
Pharmacokinetics and pharmacodynamics of figitumumab, a monoclonal antibody targeting the insulin-like growth factor 1 receptor, in healthy participants., PMID:23400740
Retraction. Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab., PMID:23211971
β-Arrestin-biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor-targeting antibodies in Ewing's sarcoma., PMID:23188799
Clinical setbacks reduce IGF-1 inhibitors to cocktail mixers., PMID:23051797
[Current status and perspectives of individualized therapy for non-small cell lung cancer based on molecular targeting]., PMID:22883466
Identification of common and distinctive mechanisms of resistance to different anti-IGF-IR agents in Ewing's sarcoma., PMID:22798295
Insulin-like growth factor receptor inhibitors: baby or the bathwater?, PMID:22761272
A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer., PMID:22553344
Heterodimerization of glycosylated insulin-like growth factor-1 receptors and insulin receptors in cancer cells sensitive to anti-IGF1R antibody., PMID:22438913
Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls., PMID:22423264
Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02)., PMID:22234739
Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft., PMID:22184402