Catalog No.
KDC29902
Description
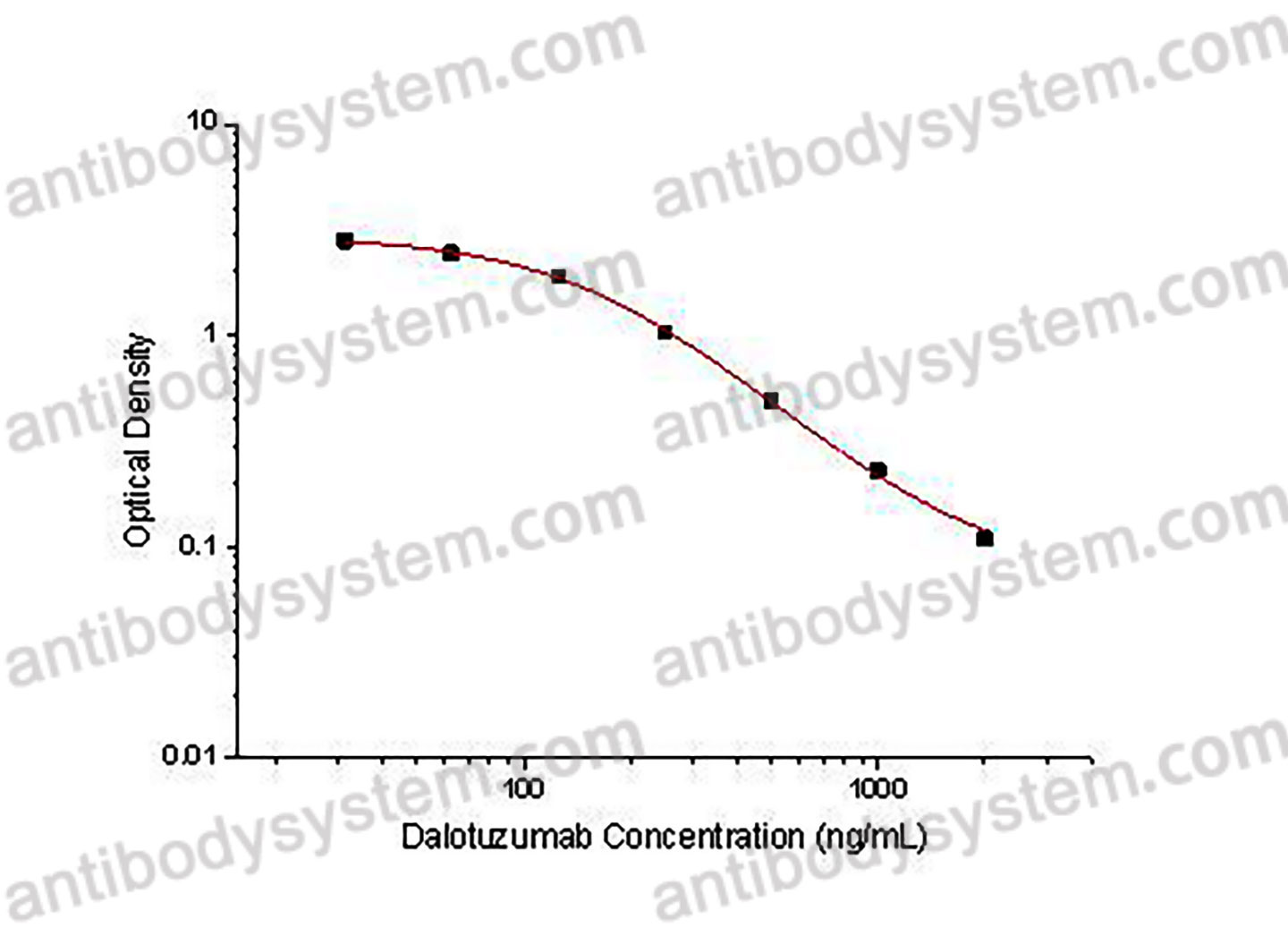
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD221 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Dalotuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Dalotuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Dalotuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Dalotuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
51.47 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1102.7
|
262.3
|
79.0
|
920.3
|
231.7
|
49.6
|
|
Standard deviation
|
83.6
|
15.5
|
6.1
|
60.8
|
13.6
|
9.6
|
|
CV (%)
|
7.6
|
5.9
|
7.7
|
6.6
|
5.9
|
19.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MK-0646, CAS: 1005389-60-5
Background
Dalotuzumab (MK-0646; h7C10), being developed by Merck & Co Inc under license from Pierre Fabre SA, is a recombinant humanized IgG1 mAb against the IGFR1 for the potential intravenous treatment of cancer. Preclinical studies have demonstrated that dalotuzumab acts by inhibiting IGF-1- and IGF-2-mediated tumor cell proliferation, IGFR1 autophosphorylation and Akt phosphorylation. In multiple cancer cell lines and in mouse xenograft models, dalotuzumab displayed significant antitumor activity, in particular against NSCLC and breast cancer. In addition, coadministration of dalotuzumab with other anticancer agents, such as taxanes, enhanced the in vitro and in vivo antitumor activity of dalotuzumab. Preliminary data from phase I clinical trials suggest that dalotuzumab is safe, well tolerated and significantly inhibits tumor proliferation. At the time of publication, several clinical trials evaluating dalotuzumab, alone and in combination with other anticancer agents, were ongoing in patients with various types of solid tumor and in patients with multiple myeloma. Although preliminary results appear promising, only future clinical and translational data will clarify the best clinical setting and treatment combinations for the optimal use of dalotuzumab in clinical practice.
Employing splice-switching oligonucleotides and AAVrh74.U7 snRNA to target insulin receptor splicing and cancer hallmarks in osteosarcoma., PMID:39720325
The efficacy of targeted therapy and/or immunotherapy with or without chemotherapy in patients with colorectal cancer: A network meta-analysis., PMID:39716565
Clinical research progress of ridaforolimus (AP23573, MK8668) over the past decade: a systemic review., PMID:38584599
Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma., PMID:29843755
Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies., PMID:28961486
A randomized phase II trial of ridaforolimus, dalotuzumab, and exemestane compared with ridaforolimus and exemestane in patients with advanced breast cancer., PMID:28681171
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy., PMID:28427155
A phase II study of combined ridaforolimus and dalotuzumab compared with exemestane in patients with estrogen receptor-positive breast cancer., PMID:28324268
Ridaforolimus (MK-8669) synergizes with Dalotuzumab (MK-0646) in hormone-sensitive breast cancer., PMID:27765027
Broad RTK-targeted therapy overcomes molecular heterogeneity-driven resistance to cetuximab via vectored immunoprophylaxis in colorectal cancer., PMID:27569653
Dalotuzumab in chemorefractory KRAS exon 2 mutant colorectal cancer: Results from a randomised phase II/III trial., PMID:27681944
IGF-1R and mTOR Blockade: Novel Resistance Mechanisms and Synergistic Drug Combinations for Ewing Sarcoma., PMID:27576731
Physiologically-based pharmacokinetic modeling to predict the clinical pharmacokinetics of monoclonal antibodies., PMID:27377311
Emerging antibodies for the treatment of multiple myeloma., PMID:27195659
Phase 1 study of dalotuzumab monotherapy and ridaforolimus-dalotuzumab combination therapy in paediatric patients with advanced solid tumours., PMID:27185573
Impact Study: MK-0646 (Dalotuzumab), Insulin Growth Factor 1 Receptor Antibody Combined with Pemetrexed and Cisplatin in Stage IV Metastatic Non-squamous Lung Cancer., PMID:26793618
RE: A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer., PMID:26754849
Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade., PMID:26429980
A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer., PMID:26405092
Minireview: Were the IGF Signaling Inhibitors All Bad?, PMID:26366975
Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma., PMID:26240353
Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with Non-small-cell lung cancer: a phase I/II randomized trial., PMID:25414803
Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial., PMID:25320355
A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours., PMID:25290091
Targeting tyrosine-kinases and estrogen receptor abrogates resistance to endocrine therapy in breast cancer., PMID:24979294
NCIC CTG IND.190 phase I trial of dalotuzumab (MK-0646) in combination with cisplatin and etoposide in extensive-stage small-cell lung cancer., PMID:24518092
Ezrin expression and cell survival regulation in colorectal cancer., PMID:24462708
In vivo analysis of insulin-like growth factor type 1 receptor humanized monoclonal antibody MK-0646 and small molecule kinase inhibitor OSI-906 in colorectal cancer., PMID:24173770
The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy., PMID:24033707
Phase 1 pharmacokinetic study of MK-0646 (dalotuzumab), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in combination with cetuximab and irinotecan in Japanese patients with advanced colorectal cancer., PMID:23921573
Trial watch: Monoclonal antibodies in cancer therapy., PMID:23482847
IGF1R-directed targeted therapy enhances the cytotoxic effect of chemotherapy in endometrial cancer., PMID:23402816
Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment., PMID:22573715
A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors., PMID:22437754
Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment., PMID:22042973
Evaluation of two ELISA methods to detect therapeutic anti-IGF1R antibodies in clinical study samples of dalotuzumab., PMID:21942521
The mechanism of action of the histone deacetylase inhibitor vorinostat involves interaction with the insulin-like growth factor signaling pathway., PMID:21931726
The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors., PMID:21907495
A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors., PMID:21810918
Antibody-based therapeutics to watch in 2011., PMID:21051951
Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer., PMID:20521225