Catalog No.
KDC29901
Description
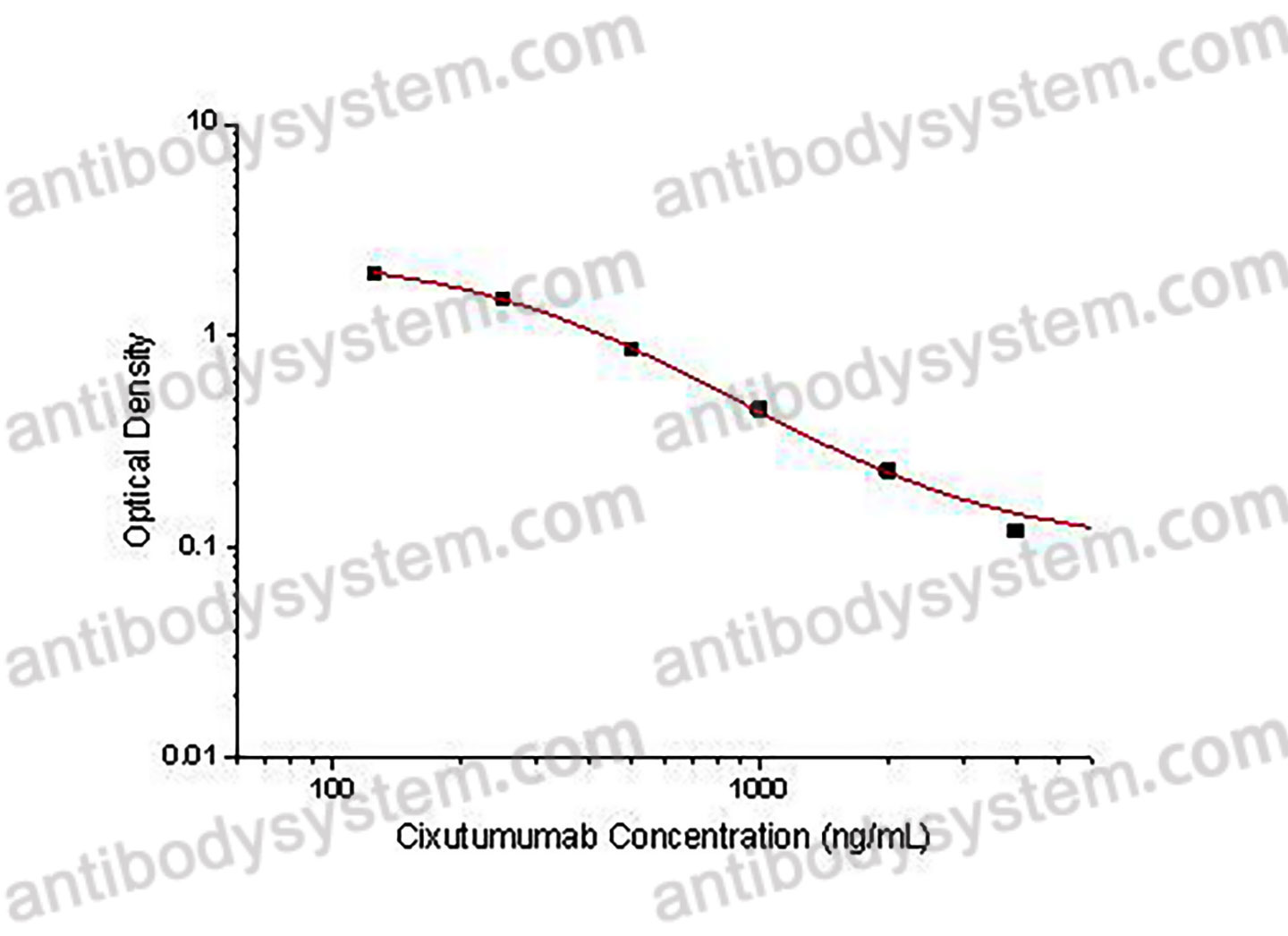
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD221 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Cixutumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Cixutumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Cixutumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Cixutumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 4,000 ng/mL
Sensitivity
39.37 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2,305.5
|
453.5
|
111.4
|
1,676.4
|
410.0
|
95.1
|
|
Standard deviation
|
245.7
|
26.4
|
10.7
|
211.0
|
32.4
|
18.8
|
|
CV (%)
|
10.7
|
5.8
|
9.6
|
12.6
|
7.9
|
19.8
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
IMC-A12, LY3012217, CAS: 947687-12-9
Background
Cixutumumab (IMC-A12) is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity for the treatment of solid tumors. This drug was developed by ImClone Systems, since acquired by Eli Lilly, using phage display technology from Dyax. Cixutumumab was well tolerated when used alone and in combination therapies, but its antitumor activity was low in the existing phase II clinical trials. An acceptable incidence of adverse effects supports further investigation of this drug, provided that it shows antitumor activity in combination with other drugs. Now, cixutumumab is involved in two cancer clinical trials, including esophageal cancer and breast cancer.
Paclitaxel With or Without Cixutumumab as Second-Line Treatment of Metastatic Esophageal or Gastroesophageal Junction Cancer: A Randomized Phase II ECOG-ACRIN Trial., PMID:37104870
Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis., PMID:36139631
Cixutumumab reveals a critical role for IGF-1 in adipose and hepatic tissue remodelling during the development of diet-induced obesity., PMID:35734881
The Immunotherapy Landscape in Adrenocortical Cancer., PMID:34071333
Randomized Phase II Trial of Capecitabine and Lapatinib with or without IMC-A12 (Cituxumumab) in Patients with HER2-Positive Advanced Breast Cancer Previously Treated with Trastuzumab and Chemotherapy: NCCTG N0733 (Alliance)., PMID:33852121
An expanded genetic code facilitates antibody chemical conjugation involving the lambda light chain., PMID:33561746
Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer., PMID:33122707
The Receptor Tyrosine Kinase RON and Its Isoforms as Therapeutic Targets in Ewing Sarcoma., PMID:32272784
A Phase I Trial of IGF-1R Inhibitor Cixutumumab and mTOR Inhibitor Temsirolimus in Metastatic Castration-resistant Prostate Cancer., PMID:32057715
Survival outcomes and risk group validation from SWOG S0925: a randomized phase II study of cixutumumab in new metastatic hormone-sensitive prostate cancer., PMID:32055002
111In- and 225Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer., PMID:31518138
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model., PMID:30726873
The addition of cixutumumab or temozolomide to intensive multiagent chemotherapy is feasible but does not improve outcome for patients with metastatic rhabdomyosarcoma: A report from the Children's Oncology Group., PMID:30351457
Randomized phase II trial of cixutumumab alone or with cetuximab for refractory recurrent/metastatic head and neck squamous cell carcinoma., PMID:29909907
A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma., PMID:29520435
Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer., PMID:29105802
Phase II randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: a trial of the ECOG-ACRIN Cancer Research Group (E3508)., PMID:28950351
A meta-analysis of CXCL12 expression for cancer prognosis., PMID:28535157
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy., PMID:28427155
In silico prediction of B cell epitopes of the extracellular domain of insulin-like growth factor-1 receptor., PMID:28261624
Adverse Events and Efficacy of Cixutumumab in Phase II Clinical Trials: A systematic Review and Meta-Analysis., PMID:27858328
Mortality Among Men with Advanced Prostate Cancer Excluded from the ProtecT Trial., PMID:27720537
An Open-Label, Multicenter, Randomized, Phase II Study of Cisplatin and Pemetrexed With or Without Cixutumumab (IMC-A12) as a First-Line Therapy in Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer., PMID:27464970
The TGFβ pathway stimulates ovarian cancer cell proliferation by increasing IGF1R levels., PMID:27299695
Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508)., PMID:27163943
A phase I study evaluating cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, given every 2 or 3 weeks in Japanese patients with advanced solid tumors., PMID:27139036
Electrolyte disorders associated with the use of anticancer drugs., PMID:26939882
STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody., PMID:26465273
Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade., PMID:26429980
Clinical and Translational Results of a Phase II, Randomized Trial of an Anti-IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy., PMID:26324738
Intrinsic Resistance to Cixutumumab Is Conferred by Distinct Isoforms of the Insulin Receptor., PMID:26263910
A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer., PMID:26082390
The Prevalence and Impact of Hyperglycemia and Hyperlipidemia in Patients With Advanced Cancer Receiving Combination Treatment With the Mammalian Target of Rapamycin Inhibitor Temsirolimus and Insulin Growth Factor-Receptor Antibody Cixutumumab., PMID:26054632
Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review., PMID:26043220
Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors., PMID:25900182
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model., PMID:25858498
SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer., PMID:25847934
Targeted therapy for thymic epithelial tumors: a new horizon? Review of the literature and two cases reports., PMID:25832879
Do thymic malignancies respond to target therapies?, PMID:25754373
Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors., PMID:25749986
Three-arm, randomized, phase 2 study of carboplatin and paclitaxel in combination with cetuximab, cixutumumab, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: An Eastern Cooperative Oncology Group (ECOG) study (E4508)., PMID:25740387
Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms., PMID:25543125
A Phase I Study of Cixutumumab (IMC-A12) in Combination with Temsirolimus (CCI-779) in Children with Recurrent Solid Tumors: A Children's Oncology Group Phase I Consortium Report., PMID:25467181
Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group., PMID:25446280
A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours., PMID:25268371
Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727)., PMID:25041791
The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial., PMID:24849545
Novel biologic therapies for thymic epithelial tumors., PMID:24847446
Incidence of mucositis in patients treated with temsirolimus-based regimens and correlation to treatment response., PMID:24668327
Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance--implications for IGF-II and IGF-IR-targeted therapy., PMID:24599933