Catalog No.
KDC15801
Description
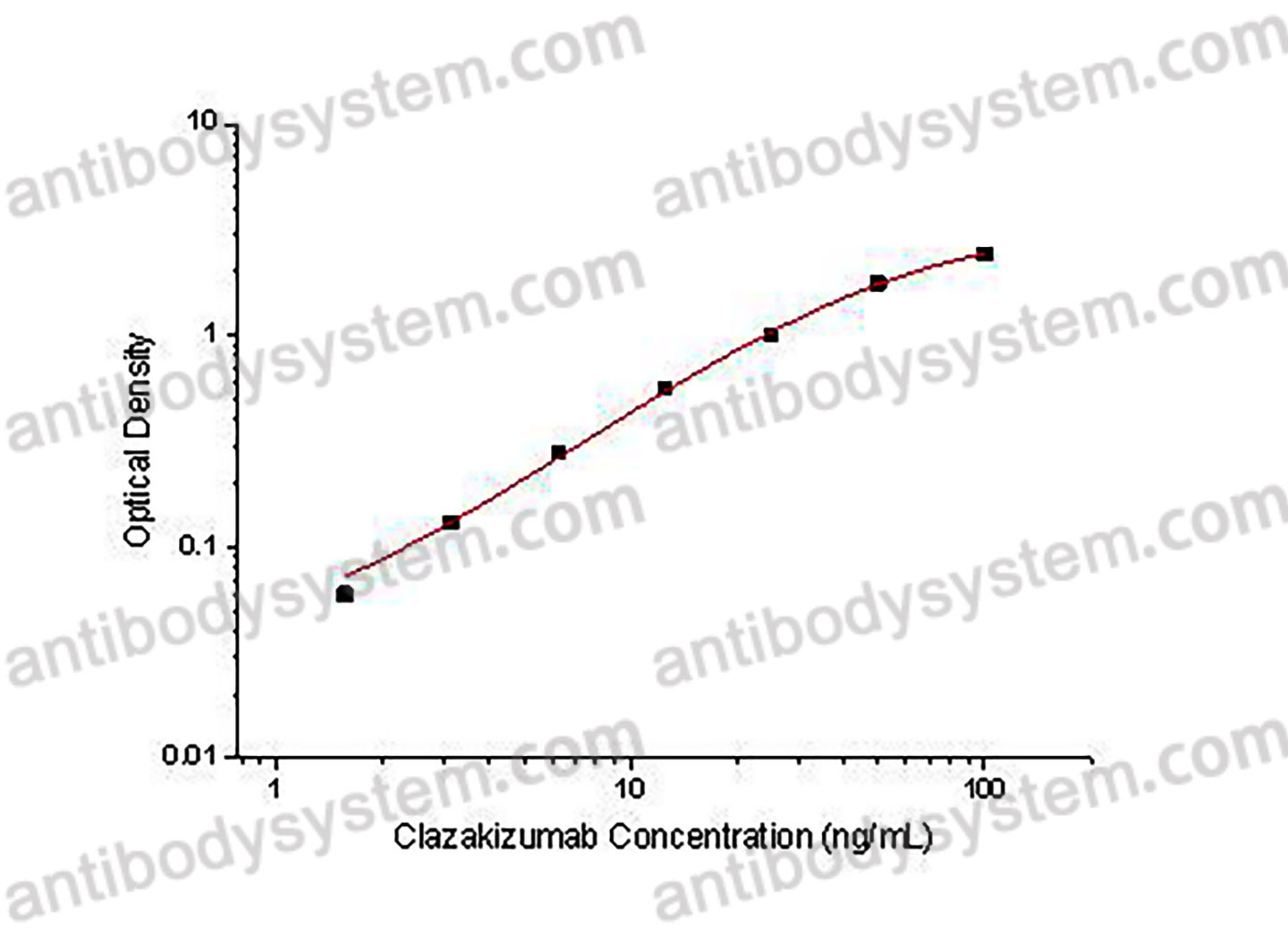
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human IL6 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Clazakizumab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Clazakizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Clazakizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.54 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
44.20
|
11.09
|
2.72
|
54.04
|
11.29
|
2.83
|
|
Standard deviation
|
4.01
|
0.60
|
0.23
|
6.41
|
0.64
|
0.18
|
|
CV (%)
|
9.06
|
5.39
|
8.47
|
11.87
|
5.66
|
6.46
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ALD-518, BMS-945429, CAS: 1236278-28-6
Background
Clazakizumab, a humanized anti–IL-6 mAb, is used in the phase II clinical trial for the treatment of RA patients. It has also been evaluated for the treatment of non-small cell lung cancer (NSCLC) and patients with head and neck cancer.
Targeting IL-6 in antibody-mediated kidney transplant rejection., PMID:40357502
[The highlights of dialysis in 2024]., PMID:40208672
Effects of Clazakizumab on Anemia and Iron Metabolism in Patients with Kidney Failure., PMID:39908111
Network meta-analysis of pharmacological treatment for antibody-mediated rejection after organ transplantation., PMID:39726594
New Therapies for Highly Sensitized Patients on the Waiting List., PMID:38995690
Author Correction: IL-6 inhibition with clazakizumab in patients receiving maintenance dialysis: a randomized phase 2b trial., PMID:38961226
Granulomatous Tubulointerstitial Nephritis in a Kidney Allograft: Treatment with Interleukin-6 Receptor Antagonist Stabilises Kidney Function., PMID:38929956
IL-6 inhibition with clazakizumab in patients receiving maintenance dialysis: a randomized phase 2b trial., PMID:38796655
Advances in desensitization for human leukocyte antigen incompatible kidney transplantation., PMID:38088373
Chronic Active Antibody-mediated Rejection: Opportunity to Determine the Role of Interleukin-6 Blockade., PMID:37941113
Interleukin-6 blocking agents for treating COVID-19: a living systematic review., PMID:37260086
Clazakizumab for the treatment of chronic active antibody-mediated rejection (AMR) in kidney transplant recipients: Phase 3 IMAGINE study rationale and design., PMID:36550562
Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts., PMID:36453709
Anti-interleukin-6 Antibody Clazakizumab in Antibody-mediated Kidney Transplant Rejection: Effect on Donor-derived Cell-free DNA and C-X-C Motif Chemokine Ligand 10., PMID:36382130
[Improving access to kidney transplantation for highly sensitized patients: What place for IL-6 pathway blockade in desensitization protocols?]., PMID:36328901
Investigational drugs for the treatment of kidney transplant rejection., PMID:36175360
Anti-interleukin-6 Antibody Clazakizumab in Antibody-mediated Renal Allograft Rejection: Accumulation of Antibody-neutralized Interleukin-6 Without Signs of Proinflammatory Rebound Phenomena., PMID:35969004
Drug discovery in spinal cord injury-induced osteoporosis: a text mining-based study., PMID:35957736
Pharmacotherapeutic options for the prevention of kidney transplant rejection: the evidence to date., PMID:35835450
Emerging drugs for antibody-mediated rejection after kidney transplantation: a focus on phase II & III trials., PMID:35715978
A Randomized Double-Blinded Placebo Controlled Trial of Clazakizumab for the Treatment of COVID-19 Pneumonia With Hyperinflammation., PMID:35583232
Evaluation of Clazakizumab (Anti-Interleukin-6) in Patients With Treatment-Resistant Chronic Active Antibody-Mediated Rejection of Kidney Allografts., PMID:35497778
Disparities in Access to Preemptive Repeat Kidney Transplant: Still Missing the Mark?, PMID:35368561
Anti-Interleukin 6 Therapeutics for Chronic Antibody-Mediated Rejection In Kidney Transplant Recipients., PMID:34981708
Clazakizumab for desensitization in highly sensitized patients awaiting transplantation., PMID:34910841
Tocilizumab and Desensitization in Kidney Transplant Candidates: Personal Experience and Literature Review., PMID:34640377
Anti-interleukin-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection: effect on cytochrome P450 drug metabolism., PMID:34153143
IL-6 Directed Therapy in Transplantation., PMID:34099967
Interleukin-6 blocking agents for treating COVID-19: a living systematic review., PMID:33734435
Immune Responses to SARS-CoV-2 in Solid Organ Transplant Recipients., PMID:33688459
A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection., PMID:33443079
New concepts in chronic antibody-mediated kidney allograft rejection: prevention and treatment., PMID:33315763
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial., PMID:33115543
Role of monoclonal antibody drugs in the treatment of COVID-19., PMID:33083387
Outpatient management of kidney transplant recipients with suspected COVID-19-Single-center experience during the New York City surge., PMID:32578324
Successful Treatment of Severe COVID-19 Pneumonia With Clazakizumab in a Heart Transplant Recipient: A Case Report., PMID:32563584
Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis., PMID:32381564
Interleukin-6 inhibition in the management of non-infectious uveitis and beyond., PMID:31523783
Comparative efficacy and safety of targeted DMARDs for active psoriatic arthritis during induction therapy: A systematic review and network meta-analysis., PMID:31272807
Clazakizumab in late antibody-mediated rejection: study protocol of a randomized controlled pilot trial., PMID:30635033
The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer., PMID:30372844
Efficacy of new treatments for dactylitis of psoriatic arthritis: update of literature review., PMID:30328022
Anti-cytokines in the treatment of cancer cachexia., PMID:30180740
Rheumatoid arthritis: new monoclonal antibodies., PMID:29771256
Efficacy and safety of biologics targeting interleukin-6, -12/23 and -17 pathways for peripheral psoriatic arthritis: a network meta-analysis., PMID:29244162
Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology., PMID:29185959
A Systematic Review and Meta-analysis of Efficacy and Safety of Novel Interleukin Inhibitors in the Management of Psoriatic Arthritis., PMID:28926467
Immunotherapeutic Interleukin-6 or Interleukin-6 Receptor Blockade in Cancer: Challenges and Opportunities., PMID:28707587
Profile of sarilumab and its potential in the treatment of rheumatoid arthritis., PMID:28579757
EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update., PMID:28264816