Catalog No.
KDC09602
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD340 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Pertuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Pertuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Pertuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Pertuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
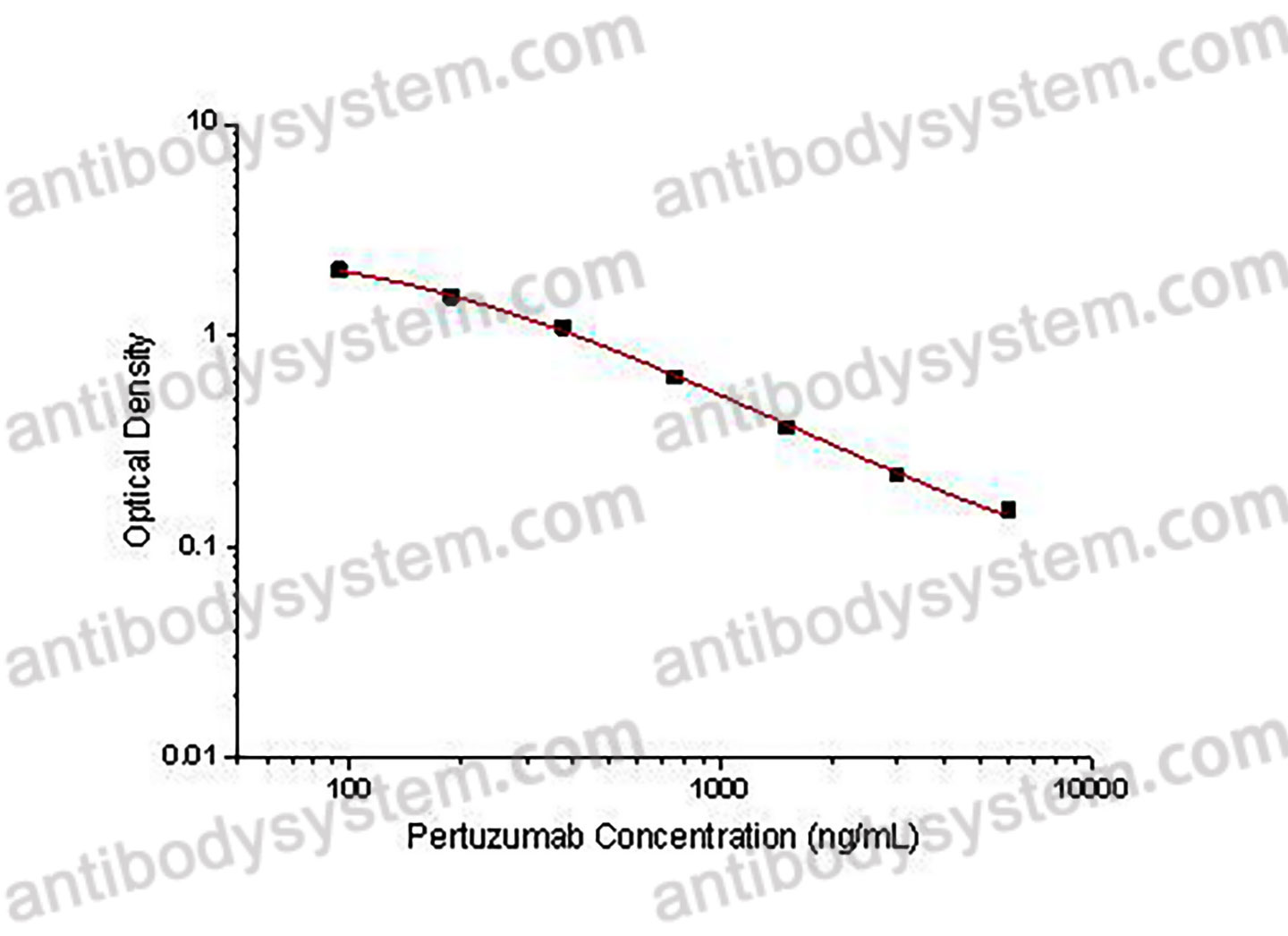
Range
93.75 - 6,000 ng/mL
Sensitivity
26.04 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
3,894.6
|
864.4
|
193.5
|
3,865.5
|
892.3
|
212.4
|
|
Standard deviation
|
232.9
|
60.4
|
17.1
|
377.5
|
60.2
|
9.6
|
|
CV (%)
|
6.0
|
7.0
|
8.8
|
9.8
|
6.7
|
4.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
OMNITARG, rhuMAB 2C4, CAS: 380610-27-5
Background
Pertuzumab (also called 2C4, trade name Perjeta) is a humanized (from mouse) monoclonal antibody (mAb) used in combination with trastuzumab and docetaxel for the treatment of metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer. It was discovered and developed by Genentech, a subsidiary of Roche, and was first approved in 2012. It is manufactured recombinantly in Chinese hamster ovary (CHO) cells. The monoclonal antibody 2C4 appears to have first been published in 1990 by scientists from Genentech. By 2003 Genentech understood that 2C4 prevented HER2 dimerizing with other HER receptors and had begun Phase I trials, aiming for a broad range of cancers, not just one’s overexpressing HER2. It was the first known HER dimerization inhibitor. In 2005 Genentech presented poor results of Phase II trials of pertuzumab as a single agent in prostate, breast, and ovarian cancers, and said that it intended to continue developing it in combination with other drugs for ovarian cancer. In 2007 Genentech dropped the trade name Omnitarg. In 2012 the results were published of the CLEOPATRA trial, a randomized placebo-controlled Phase III trial of pertuzumab in combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer. Pertuzumab received US Food and Drug Administration (FDA) approval for the treatment of HER2-positive metastatic breast cancer later that year. The FDA approved the neoadjuvant indication in 2013. Pertuzumab was approved in Europe in 2013.
Medicare spending and use of subcutaneous biologic formulations with hyaluronidase., PMID:40515473
Pharmacokinetic bioequivalence of the fixed-dose combination of pertuzumab and trastuzumab administered subcutaneously using a handheld syringe or an on-body delivery system., PMID:40514611
ctDNA Analysis in ERBB2-Amplified Colorectal Cancer: Biomarker Analysis of the MyPathway Trial., PMID:40512191
Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer in first-line/second-line setting., PMID:40510155
Long and short-term efficacy and safety comparison of nab-paclitaxel versus paclitaxel combined with trastuzumab and pertuzumab for neoadjuvant treatment of HER2-positive breast cancer: A systematic review and meta-analysis., PMID:40493995
A Randomized Phase 2 Study of Neratinib With or Without Fulvestrant for Patients With HER2-Positive, Estrogen Receptor-Positive Metastatic Breast Cancer., PMID:40484776
Advancing neoadjuvant therapy with inetetamab for HER2-Positive breast cancer., PMID:40482910
Spatial discovery of pyrotinib overcoming HER2-positive breast cancer resistance by breaking fibroblast-induced immune barriers., PMID:40480079
Continuous glucose monitoring to characterize hyperglycemia during chemotherapy for early stage breast cancer., PMID:40468140
Long-Term Safety and Efficacy of the Fixed-Dose Combination of Pertuzumab and Trastuzumab for Subcutaneous Injection in Patients With HER2-Positive Early Breast Cancer in PHranceSCa, a Randomized, Open-Label Phase II Study., PMID:40461387
Signal Mining and Analysis of Drug-Induced Myelosuppression: A Real-World Study From FAERS., PMID:40439714
Male Occult Primary Breast Cancer Diagnosed with Small Bowel Metastases: A Case Report., PMID:40433060
HER2-Positive Breast Cancer-Current Treatment Management and New Therapeutic Methods for Brain Metastasis., PMID:40426980
Cardiotoxic Effects of HER2-Targeted Therapies: Insights From a Retrospective Study at a Romanian Oncology Center., PMID:40421343
T-DM1 with concurrent radiotherapy in HER2-positive breast cancer: preclinical evaluation and mechanisms, prediction, and exploration of adverse effects., PMID:40402389
Docetaxel associated myositis., PMID:40395000
Excellent efficacy of trastuzumab deruxtecan in a patient with HER2-positive advanced breast cancer complicated by pulmonary lymphangitic carcinomatosis: a case report and literature review., PMID:40371348
Trends in the Management of Small HER2-Positive Breast Cancers., PMID:40360838
Characterization of mAb104, a Monoclonal Antibody Targeting a Conformationally Exposed, Tumor-specific epitope of HER2., PMID:40358499
Efficacy and safety of inetetamab plus pertuzumab and nab-paclitaxel as neoadjuvant therapy for HER2+ breast cancer: A single-arm multicenter phase II clinical trial., PMID:40354993
Predictive factors for pCR and relapse following neoadjuvant dual HER2-blockade in HER2+ breast cancer: an international cohort study., PMID:40348907
Prognostic impact of tumor-infiltrating lymphocytes in HER2+ metastatic breast cancer receiving first-line treatment., PMID:40346114
Comprehensive analysis of adverse event profile changes with pertuzumab addition to trastuzumab-based breast cancer therapy: Disproportionality analysis using VigiBase., PMID:40344285
Investigations of Influence of Antibody Binding Kinetics on Tumor Distribution and Anti-Tumor Efficacy., PMID:40341444
Detection of HER2-Low Lesions Using HER2-Targeted PET Imaging in Patients with Metastatic Breast Cancer: A Paired HER2 PET and Tumor Biopsy Analysis., PMID:40341092
Advancing Breast Cancer Treatment: The Role of Immunotherapy and Cancer Vaccines in Overcoming Therapeutic Challenges., PMID:40333213
Chemotherapy-free neoadjuvant pembrolizumab combined with trastuzumab and pertuzumab in HER2-enriched early breast cancer (WSG-KEYRICHED-1): a single-arm, phase 2 trial., PMID:40318646
Overall survival of patients with de Novo HER2-positive metastatic breast cancer in the Netherlands from 2008 to 2017: A population-based cohort study of systemically treated patients., PMID:40306118
Excellent Response to Trastuzumab Deruxtecan of a Large Medullary Metastasis from Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Case Report., PMID:40302988
Association of statin use on survival outcomes of patients with early-stage HER2-positive breast cancer in the APHINITY trial., PMID:40293644
Collaboration Between Palliative Care and Oncology in the Era of Personalized Medicine., PMID:40285502
Adjuvant Metronomic Chemotherapy After Surgery in pT1-T2 N0 M0 HER2-Positive and ER/PR-Positive Breast Cancer Plus Targeted Therapy, Anti-Hormonal Therapy, and Radiotherapy, with or Without Immunotherapy: A New Operational Proposal., PMID:40282499
ZMYND8 drives HER2 antibody resistance in breast cancer via lipid control of IL-27., PMID:40281007
HER2DX ERBB2 mRNA score in first-line advanced HER2-positive breast cancer treated with chemotherapy, trastuzumab, and pertuzumab., PMID:40280938
Real-world comparative effectiveness and safety of Pertuzumab in patients with HER2+ metastatic breast cancer: A pan-Canadian population-based cohort study., PMID:40268528
Pyrotinib and Nab-Paclitaxel in HER2-Positive Breast Cancer (PANHER Trial): A Prospective, Single-Arm, Phase II Trial., PMID:40263948
Differences in Responses to Neoadjuvant Anti-HER2 Therapy between HER2 2+ISH+ and HER2 3+ in HER2-Positive Breast Cancer., PMID:40259803
Impact of Hormone Receptor Status and HER2 Expression on Neoadjuvant Targeted Therapy Response in HER2-Positive Breast Cancer: A Multicenter Retrospective Study., PMID:40246081
Safety and Efficacy of Anti-Human Epidermal Growth Factor 2 Agents in the Treatment of Biliary Tract Cancers: A Systematic Review., PMID:40239136
Erratum: Pertuzumab Retreatment for Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced/Metastatic Breast Cancer (PRECIOUS Study): Final Overall Survival Analysis., PMID:40215433
Comparison of paclitaxel and docetaxel in dual HER2 blockade: efficacy and safety in neoadjuvant treatment of HER2-positive breast cancer., PMID:40214840
Treatment patterns and associated outcomes among patients with HER2+ metastatic breast cancer in the United States: an observational cohort study., PMID:40192324
[A Case of Pulmonary Tuberculosis Developed During Neoadjuvant Chemotherapy for HER2-Enriched Breast Cancer]., PMID:40189766
In vitro and in vivo evaluation of anti-HER2 antibody conjugates labelled with 225Ac., PMID:40183827
Multiplex Spatial Proteomic Analysis of HER2-Positive Breast Tumors Reveals Unique Molecular and Immunologic Features Associated With Treatment Response., PMID:40179327
Pyrotinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (PERSIST): A multicenter phase II trial., PMID:40178527
Disproportionality analysis of interstitial lung disease associated with novel antineoplastic agents during breast cancer treatment: a pharmacovigilance study., PMID:40166653
Real-life data on adjuvant trastuzumab emtansine treatment in early-stage HER2-positive breast cancer: a Turkish Oncology Group study., PMID:40162996
Real-Life Experience of HER2 (Human Epidermal Growth Factor Receptor 2)-Positive Advanced Breast Cancer Patients Treated With T-DXd (Trastuzumab Deruxtecan): A Multicentric Portuguese Study., PMID:40161117
Unraveling the Crimson puzzle: Two case reports/case series of hemorrhagic cystitis after combination chemotherapy with docetaxel, carboplatin, trastuzumab and pertuzumab in breast cancer., PMID:40153764