Catalog No.
KDC08001
Description
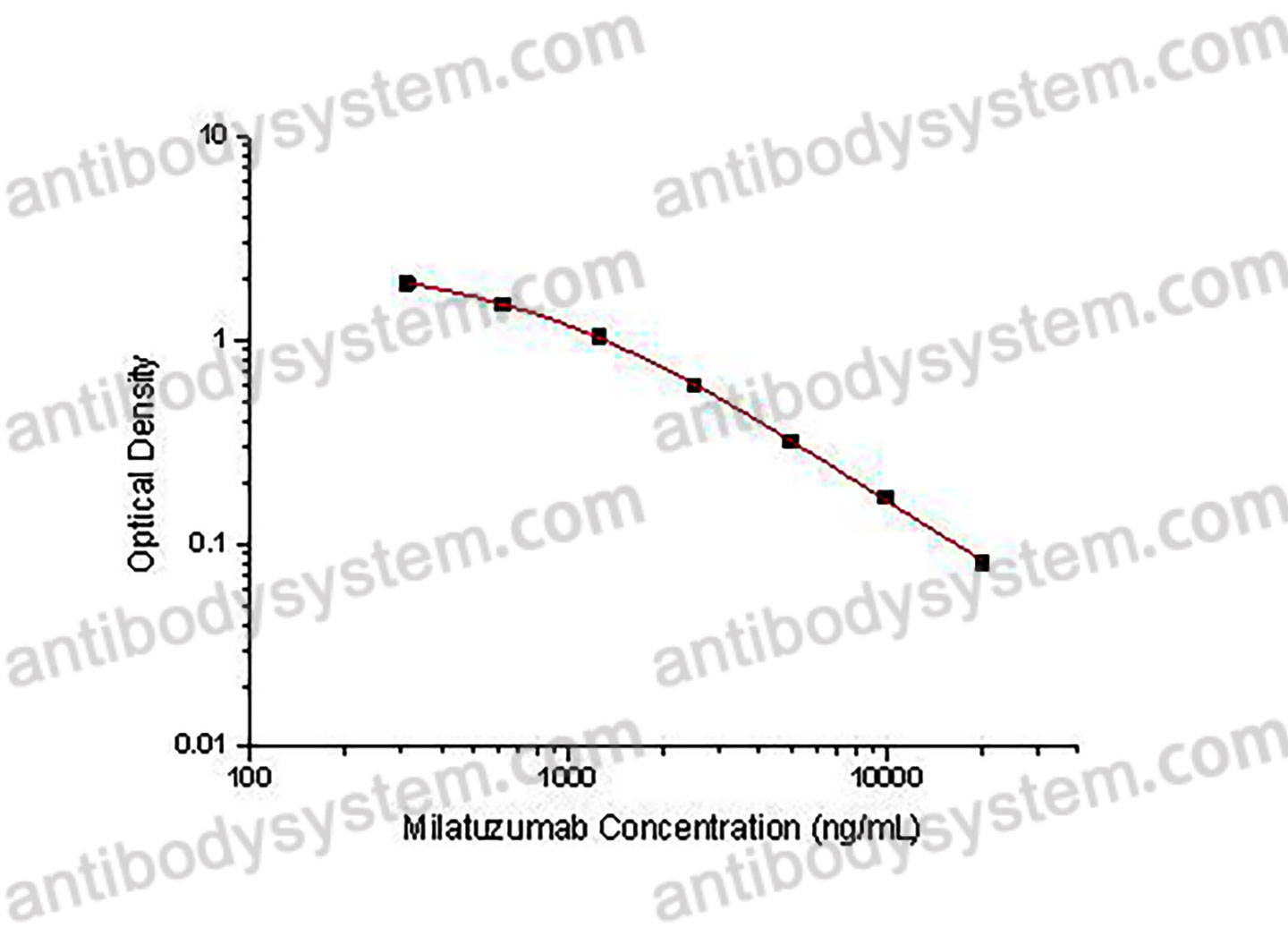
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD74 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Milatuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Milatuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Milatuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Milatuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
312.5 - 20,000 ng/mL
Sensitivity
107.29 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
7996.8
|
1772.3
|
383.0
|
10255.9
|
2188.2
|
473.4
|
|
Standard deviation
|
738.2
|
122.6
|
42.4
|
1122.0
|
266.6
|
50.4
|
|
CV (%)
|
9.2
|
6.9
|
11.1
|
10.9
|
12.2
|
10.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CD74-DOX (ADC), MEDI-115, hLL1, hLL1-DOX (ADC), CAS: 899796-83-9
Background
Milatuzumab is a new immunotherapeutic agent targeting CD74, a membrane protein preferentially expressed in hematopoietic cancers and some solid tumors. Broad expression and fast internalization makes CD74 an ideal target for cancer therapy. We reviewed published articles about CD74 and milatuzumab. We present a comprehensive review of CD74 functions and provide explanation of milatuzumab antitumor effects. This review describes CD74 protein biology with the emphasis on the role of CD74 in tumor survival and its new role in regulation of the Fas death receptor. The development of CD74 targeting therapies to induce tumor regression and cancer cell apoptosis is described and results of clinical trials are discussed. Milatuzumab shows selective binding and rapid internalization into CD74-positive cancer cells. Milatuzumab with and without conjugated toxins synergizes with other chemotherapeutic agents and elicits significant antitumor effects in mice. In a Phase I trial, milatuzumab showed no severe adverse effects in patients with relapsed/refractory multiple myeloma and it stabilized the disease in some patients for up to 12 weeks. Ongoing trials testing different treatment schedules of milatuzumab in chronic lymphocytic leukemia, non-Hodgkin's lymphoma and multiple myeloma indicate that milatuzumab shows no severe adverse effects in humans.
A novel CAR-T cell product targeting CD74 is an effective therapeutic approach in preclinical mantle cell lymphoma models., PMID:37740214
The first ADC bearing the ferroptosis inducer RSL3 as a payload with conservation of the fragile electrophilic warhead., PMID:36334452
Experience with milatuzumab, an anti-CD74 antibody against immunomodulatory macrophage migration inhibitory factor (MIF) receptor, for systemic lupus erythematosus (SLE)., PMID:33619162
Emerging immune targets for the treatment of multiple myeloma., PMID:29421983
A phase I-II clinical trial of the anti-CD74 monoclonal antibody milatuzumab in frail patients with refractory chronic lymphocytic leukaemia: A patient based approach., PMID:28466956
The European League Against Rheumatism (EULAR) - 17th Annual European Congress of Rheumatology (June 8-11, 2016 - London, UK)., PMID:27458612
Emerging antibodies for the treatment of multiple myeloma., PMID:27195659
Management of Multiple Myeloma with Second-Generation Antibody-Drug Conjugates., PMID:26927803
ROR1-targeted delivery of OSU-2S, a nonimmunosuppressive FTY720 derivative, exerts potent cytotoxicity in mantle-cell lymphoma in vitro and in vivo., PMID:25937048
The combination of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, demonstrates activity in patients with relapsed and refractory B-cell non-Hodgkin lymphoma., PMID:25847298
Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas., PMID:25754579
CD74 interferes with the expression of fas receptor on the surface of lymphoma cells., PMID:25304249
Current Phase II antibody-drug conjugates for the treatment of lymphoid malignancies., PMID:24708159
Milatuzumab and veltuzumab induce apoptosis through JNK signalling in an NF-κB dependent human transformed follicular lymphoma cell line., PMID:24386925
Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma., PMID:24112026
CD84 is a survival receptor for CLL cells., PMID:23435417
Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers., PMID:23427296
Milatuzumab-conjugated liposomes as targeted dexamethasone carriers for therapeutic delivery in CD74+ B-cell malignancies., PMID:23209030
Prevention of acute graft-versus-host disease in a xenogeneic SCID mouse model by the humanized anti-CD74 antagonistic antibody milatuzumab., PMID:23025988
CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder., PMID:22905972
The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression., PMID:22404985
FTY720-induced blockage of autophagy enhances anticancer efficacy of milatuzumab in mantle cell lymphoma: is FTY720 the next autophagy-blocking agent in lymphoma treatment?, PMID:22377620
Novel targeted therapies for mantle cell lymphoma., PMID:22361516
Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas., PMID:22271448
FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death., PMID:22042694
The secret second life of an innocent chaperone: the story of CD74 and B cell/chronic lymphocytic leukemia cell survival., PMID:21417823
Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma., PMID:21228331
Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells., PMID:20574049
Milatuzumab - a promising new immunotherapeutic agent., PMID:19968579
Gateways to clinical trials., PMID:19455266
Combining milatuzumab with bortezomib, doxorubicin, or dexamethasone improves responses in multiple myeloma cell lines., PMID:19351768
Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma., PMID:19223402
Milatuzumab: a promising new agent for the treatment of lymphoid malignancies., PMID:19053886
CD74: a new candidate target for the immunotherapy of B-cell neoplasms., PMID:17875789