Catalog No.
KDB95801
Description
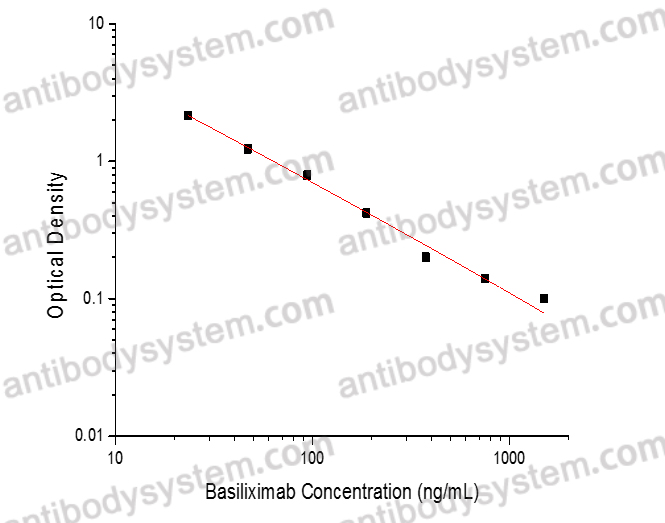
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD25 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Basiliximab in the sample competitively binds to the pre-coated protein with biotin-labeled Basiliximab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Basiliximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Basiliximab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
23.44 - 1,500 ng/mL
Sensitivity
13.31 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
810.6
|
179.7
|
38.1
|
703.2
|
169.8
|
41.3
|
|
Standard deviation
|
91.3
|
16.6
|
4.7
|
110.2
|
15.8
|
5.6
|
|
CV (%)
|
11.3
|
9.2
|
12.3
|
15.7
|
9.3
|
13.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CHI621, CHI-621 / SDZ-CHI-621, CAS: 179045-86-4
Background
Basiliximab is a chimeric anti-intcrleukin-2 receptor monoclonal antibody. Basiliximab is a glycoprotein produced by recombinant technology. It is used to prevent white blood cells from acute renal transplantation rejection. It specifically binds to and blocks the alpha chain of interleukin-2 receptors (IL-2R alpha), also known as CD25 antigen, on the surface of activated T-lymphocytes. Due to its monoclonal nature it provides safer and more predictable therapeutic, that is, immunosuppressive response of the polyclonal antibodies. The most common adverse effects in adult patients are constipation, infections, pain, nausea, peripheral oedema, hypertension, anaemia, headache, hyperkalacmia, hypercholesterolemia, increase in serum creatinine, and hypophosphataemia.
Induction With Antithymocyte Globulin Is Associated With Decreased Mortality and PTLD in Pediatric Liver Transplantation: A UNOS Data Analysis., PMID:40490923
TIM3 and TIGIT-expressing CD4 T cells are impacted by kidney transplantation and associated with risk of infection., PMID:40475763
Incidence of catheter-related bloodstream infection (CRBSI) in immunosuppressed hosts post solid organ transplant (SOT): a single center experience., PMID:40438131
Inefficiency Rates of Biological Immunosuppressive Induction Agents Used in Organ Transplantation: A Pharmacovigilance Study., PMID:40429403
The first live term birth following uterus transplantation in Australia., PMID:40406819
Infection Risks With Thymoglobulin Use for Delayed Graft Function in Deceased Donor Kidney Transplantation: Research Letter., PMID:40352746
Efficacy and Safety of Induction Therapy in Kidney Transplant Recipients: A Propensity Score Matching Analysis in a Multicenter Retrospective Observational Study., PMID:40312217
Optimization of the Tacrolimus Concentration-to-Dose Ratio Cut-Off Value to Define Metabolism Groups., PMID:40283373
Machine Learning Model for Predicting Biliary Complications After Liver Transplantation., PMID:40249076
Evaluation of biological therapies in autoimmune hepatitis: A case-based systematic review., PMID:40123748
Basiliximab for IL-2-Associated Inflammatory Disorder With Atopic Dermatitis., PMID:40000115
Acute Respiratory Distress Syndrome in Young Postrenal Transplant Patients Receiving Basiliximab., PMID:39996094
Evaluating the efficacy of basiliximab versus no induction in low-immunological-risk kidney transplant recipients: a propensity score matched analysis., PMID:39978365
Induction outcomes in adult kidney transplantation: Two decades of UNOS data analysis., PMID:39947488
Patterns of belatacept use and risk of post-transplant lymphoproliferative disorder in US kidney transplant recipients: An analysis of the Organ Procurement and Transplantation Network database., PMID:39792912
Association Between Early Immunosuppression Center Variability and One-Year Outcomes After Pediatric Liver Transplant., PMID:39777775
Assessment of respiratory function in children after kidney transplantation., PMID:39775938
Identifying Promising Immunomodulators for Type 1 Diabetes (T1D) and Islet Transplantation., PMID:39735417
Rejection in the setting of combined Heart and Liver Transplantation., PMID:39713488
Alemtuzumab Associated With Higher Mortality Than Basiliximab in Older Kidney Transplant Recipients., PMID:39708389
The Use of Long-Term Monthly Basiliximab Infusions as Rescue Maintenance Immunosuppression in Pancreas Transplant Recipients., PMID:39688531
First ABO-Incompatible Pediatric Kidney Transplant in South Africa-A Case Report., PMID:39688284
Combined Cytokine Blockade Therapy (CCBT) Using Basiliximab and Infliximab for Treatment of Steroid-Refractory Graft-Versus-Host Disease (SR-GvHD)., PMID:39682101
Advancing kidney transplantation in black patients: a genetics-based and personalized approach under NICE, KDIGO, and ERBP guidelines., PMID:39676231
Basiliximab is superior to low dose rabbit anti-thymocyte globulin in pediatric kidney transplant recipients: The younger, the better., PMID:39658242
Basiliximab induction immunosuppression and lung transplant outcomes: Propensity analysis in a multicenter cohort., PMID:39642952
The role of induction therapy in lung transplantation., PMID:39551266
Retrospective Cohort Study of Associated Factors for Intestinal Complications in Pediatric Liver Transplantation., PMID:39526469
Beyond Borders: International Immunosuppression Practices in Pediatric Lung Transplantation., PMID:39520098
Pediatric kidney transplantation in Europe, a clinical snapshot pilot., PMID:39513158
European Association of Urology Guidelines on Renal Transplantation: Update 2024., PMID:39489684
Basiliximab vs. No Induction Therapy in Kidney Transplant Recipients with a Low Immunological Risk Profile Receiving Tacrolimus/Mycophenolate/Steroids Maintenance Immunosuppression., PMID:39458101
Evaluation of Single Versus Two-Dose Basiliximab Induction Therapy in Live-Donor Liver Transplant., PMID:39436115
Haploidentical hematopoietic stem cell transplantation for hematologic malignancies: a novel conditioning regimen with anti-T lymphocyte immunoglobulin instead of anti-thymocyte globulin for in vivo T cell depletion., PMID:39402188
Clinical profile and outcome of kidney transplantation at Muhimbili National Hospital, Tanzania., PMID:39342167
Basiliximab induction alone vs a dual ATG-basiliximab approach in first live-donor non-sensitized kidney transplant recipients with low HLA matching., PMID:39314868
Intraoperative Mean Arterial Pressure and Postoperative Delayed Graft Function in Kidney Transplantation: Evaluating Three Commonly Used Thresholds., PMID:39302234
Safety assessment of basiliximab using real-world adverse event data from the FDA Adverse Event Reporting System Database: A retrospective observational study., PMID:39252278
Post-Kidney Transplant Cancer: A Real-World Retrospective Analysis From a Single Italian Center., PMID:39228659
Reversible cerebral vasoconstriction syndrome post-cardiac transplantation: a therapeutic dilemma: case report., PMID:39123195
Early acute kidney injury after tacrolimus administration in heart transplant recipients receiving basiliximab induction therapy., PMID:39120080
Role of Induction in a Haplomatch, Related, Low-Risk, Living-Donor Kidney Transplantation with Triple Drug Immunosuppression: A Single-Center Study., PMID:39114397
Contemporary Immunosuppression Management and 1-Year Outcomes in Dual Organ Heart Transplantation., PMID:39113661
Low-Dose Thymoglobulin versus Basiliximab Induction Therapy in Low-Risk Living Related Kidney Transplant Recipients: Three-Year Follow-Up Study., PMID:39079480
Efficacy and Safety of Bleselumab in Preventing the Recurrence of Primary Focal Segmental Glomerulosclerosis in Kidney Transplant Recipients: A Phase 2a, Randomized, Multicenter Study., PMID:39042770
Vedolizumab plus basiliximab as second-line therapy for steroid-refractory lower gastrointestinal acute graft-versus-host disease., PMID:39021571
Successful A2 to O Simultaneous Liver and Kidney Transplantation in the Setting of Pre-operative Positive HLA Crossmatch: A Case Report., PMID:39004578
Everolimus plus reduced calcineurin inhibitor prevents de novo anti-HLA antibodies and humoral rejection in kidney transplant recipients: 12-month results from the ATHENA study., PMID:38993866
Renal transplantation using kidneys from a donor with high grade cytomegalovirus viraemia: case report and literature review., PMID:38991589
Ruxolitinib Plus Basiliximab Therapy for Steroid-Refractory Acute Graft-Versus-Host Disease in Unrelated Cord Blood Transplantation: A Large-Scale Study., PMID:38971463