Catalog No.
KDB95601
Description
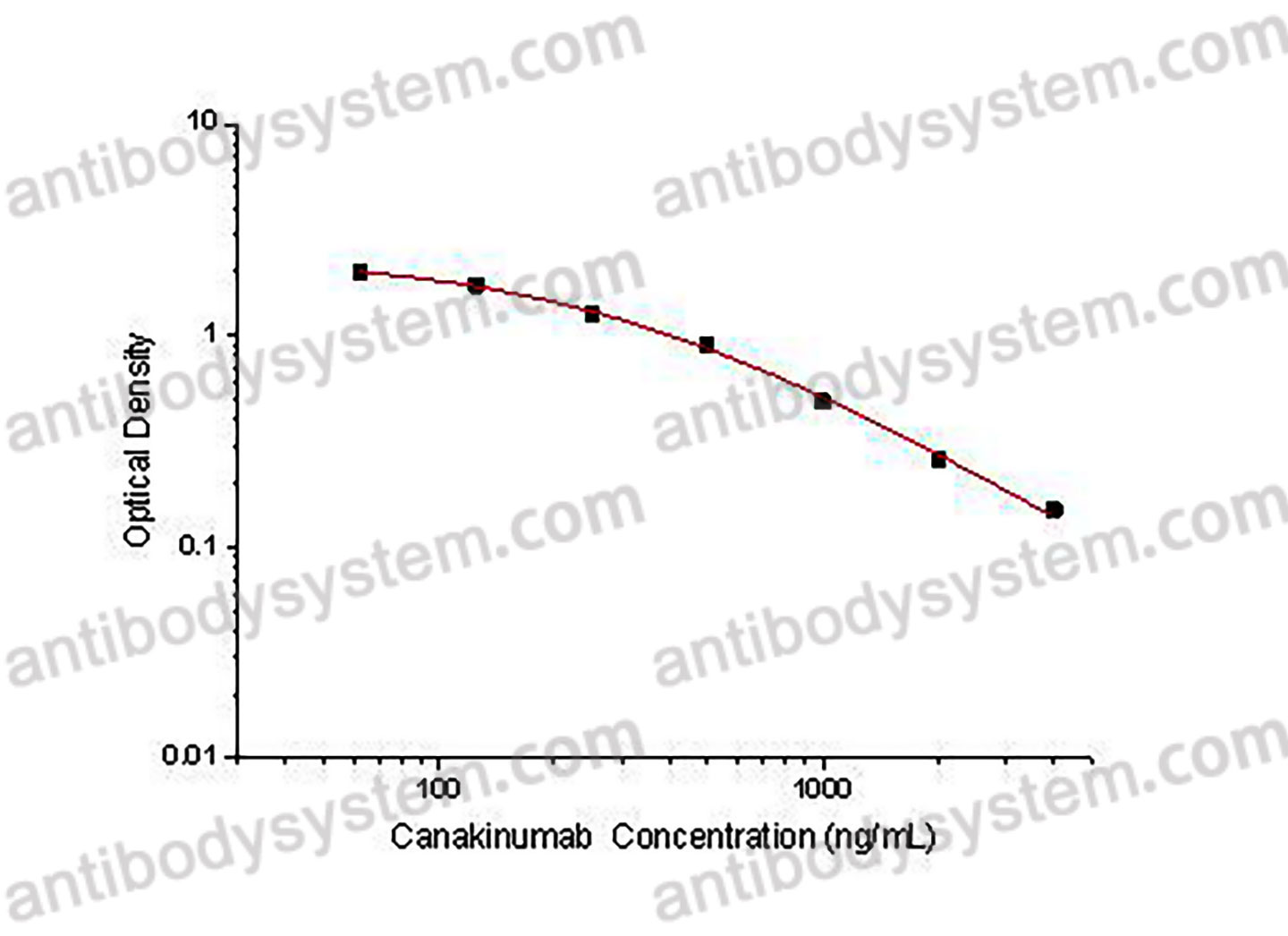
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL1B has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Canakinumab in the sample competitively binds to the pre-coated protein with biotin-labeled Canakinumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Canakinumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Canakinumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
64.37 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2059.5
|
546.7
|
246.2
|
2022.9
|
620.2
|
226.8
|
|
Standard deviation
|
136.7
|
57.8
|
34.5
|
292.1
|
109.8
|
40.9
|
|
CV (%)
|
6.6
|
10.6
|
14.0
|
14.4
|
17.7
|
18.0
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
ACZ885, CAS: 914613-48-2
Background
Canakinumab (ACZ885, Ilaris) is a human anti-IL-1β monoclonal antibody developed by Novartis. its mode of action is based on the neutralization of 1β signaling, resulting in suppression of inflammation in patients with disorders of autoimmune origin. In June 2009 the drug was approved by the US Food and Drug Administration for the treatment of familial cold auto-inflammatory syndrome and Muckle-wells syndrome, which are inflammatory diseases related to cryopyrin-associated periodic syndromes. The drug is currently being evaluated for its potential in the treatment of rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis, chronic obstructive pulmonary disease, type 1 and 2 diabetes and ocular diseases. Reports from clinical trials suggest that canakinumab is well-tolerated in most patients, and no serious adverse effects have been reported. The drug provides significant advantages over existing competitive therapies, including bimonthly administration and approved use in children.
Interleukin-1β in circulating mononuclear cells predicts steatotic liver disease improvement after weight loss in subjects with obesity and prediabetes or type 2 diabetes., PMID:40514652
Unmasking the Masquerade: A Case Report of Adult-Onset Still's Disease., PMID:40486417
Is It Time to Revisit the Role of Interleukin-1 Inhibitors in Osteoarthritis?, PMID:40458527
The first nationwide epidemiological survey of chronic recurrent multifocal osteomyelitis in Japan., PMID:40445191
Canakinumab treatment patterns in sJIA, FMF, TRAPS, and MKD/HIDS: real-world insights from a Belgian non-interventional study., PMID:40442768
Canakinumab for the treatment of postprandial hypoglycaemia: study protocol for a randomised, placebo-controlled, parallel-group, double-blind, multicentric, superiority trial-the CanpHy study., PMID:40425249
Impact of Interleukin-1 Blockade on the Development of Macrophage Activation Syndrome in Still's Disease: Incidence and Diagnostic Validity of the EULAR/ACR/PRINTO 2016 MAS Classification Criteria., PMID:40420428
Pregnancy outcomes after maternal and paternal anti-IL-1 treatment exposure in cryopyrin associated periodic syndromes (CAPS)., PMID:40411758
Diagnosis, management, and monitoring of interleukin-1 mediated diseases in Central and Eastern Europe: real-world data., PMID:40405263
Pilot study of canakinumab (Ilaris) in steroid naïve children with Duchenne muscular dystrophy demonstrates safety and exploratory changes in potential serum protein response biomarkers., PMID:40396427
Neutrophils predominate as IL1B-expressing cells in Schnitzler syndrome: Insights from the SCan study to evaluate the efficacy and safety of canakinumab in Japanese patients., PMID:40393905
Pulmonary arterial hypertension in adults with Still's disease: another pulmonary manifestation associated with HLA-DRB1*15., PMID:40379524
Targeting the NLRP3 inflammasome for inflammatory disease therapy., PMID:40374417
Post-marketing safety of anakinra and canakinumab: a real-world pharmacovigilance study based on FDA adverse event reporting system., PMID:40371323
Identification of Hub Genes and Pathways in Preinfusion Chimeric Antigen Receptor (CAR) T-cell Products Associated With Cytokine Release Syndrome., PMID:40370902
Endogenous lipoid pneumonia in adult autoinflammatory disease., PMID:40368851
Targeting IL-6 in antibody-mediated kidney transplant rejection., PMID:40357502
Current anti-inflammatory strategies for treatment of heart failure: From innate to adaptive immunity., PMID:40348101
Contemporary management paradigms and emerging therapeutics for myelodysplastic syndromes/neoplasms., PMID:40302714
Canakinumab for the management of pyoderma gangrenosum: a case series of two patients and literature review., PMID:40302177
Buzzing Away Pain: Efficacy of Buzzy® in Reducing Pain During Canakinumab Treatment for Familial Mediterranean Fever., PMID:40291186
Shared Mechanisms in Cancer and Cardiovascular Disease: S100A8/9 and the NLRP3 Inflammasome: JACC: CardioOncology State-of-the-Art Review., PMID:40260700
Long-term efficacy and safety of colchicine and anti-IL-1 blockers in FMF: results from the Eurofever multicenter observational study., PMID:40252570
Tumor Necrosis Factor Receptor-Associated Periodic Syndrome: Genetics, Autoinflammation, and Recurrent Pericarditis., PMID:40250904
Seeking and treating inflammation in ischaemic heart disease: are we ready?, PMID:40248283
Successful Treatment of PAPASH Syndrome With Concomitant FMF Using IL-1 Blockade: A Case Report., PMID:40243006
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
The Efficacy and Safety of Hepatitis A Vaccine in Children and Young Adults With an Autoinflammatory Diseases on Canakinumab and Tocilizumab Treatments: A Prospective Observational Controlled Study., PMID:40172610
First-line biological versus conventional synthetic disease-modifying antirheumatic drug therapy in adult-onset Still's disease: a multicentre, retrospective, propensity weighted cohort study., PMID:40164168
Current therapeutic options for adult patients with urticarial vasculitis: A scoping review., PMID:40157507
Use of Biologic Therapy in AA Amyloidosis Patients Undergoing Dialysis-A Systematic Literature Review., PMID:40152016
Treatment of uveitis in Blau syndrome: A systematic review and meta-analysis., PMID:40147219
The Impact of the Tumor Microenvironment on the Effect of IL-1β Blockade in NSCLC: Biomarker Analyses from CANOPY-1 and CANOPY-N Trials., PMID:40116353
Two Novel Variants in the LRR Domain of NLRP3 Causing Leukoencephalopathy: A Case Report., PMID:40116107
Use of Anti-interleukin-1 Agents in Kidney Transplant Recipients with Familial Mediterranean Fever and Amyloidosis: What have been learned so far?, PMID:40092658
The Golden Card of Interleukin-1 Blockers in Systemic Inflammasomopathies of Childhood., PMID:40076498
Deficiency of interleukin-1 receptor antagonist: A systematic review., PMID:40060136
The effect of IL-1β inhibitor canakinumab (Ilaris®) on IL-6 production in human skeletal muscle cells., PMID:40048444
Efficacy and safety profile of biotechnological agents and Janus kinase inhibitors in VEXAS syndrome: data from the international AIDA Network VEXAS registry., PMID:40046741
Successful control of recurrent MAS by canakinumab in a Sjogren syndrome patient with homozygous MEFV P369S variants, and review of literatures., PMID:40045586
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Canakinumab Treatment in Familial Mediterranean Fever Patients: With/Without Colchicine., PMID:40040547
Elevated C-reactive protein and cardiovascular risk., PMID:40014057
Balancing Effective Treatments With Potential Threats: The Impact of Biologic Agent Use on Tuberculosis Development in Children With Chronic Inflammatory Disorders., PMID:39989306
Discovery and Phase 1 study of a novel monoclonal antibody against human IL-1β for the treatment of IL-1β-mediated diseases., PMID:39964852
Blood molecular subtypes to guide precision treatment strategies in systemic juvenile idiopathic arthritis., PMID:39923112
Are participants in gout medication registration clinical trials representative of people with gout in the general population?, PMID:39919487
Associations of Serum Urate and Cardiovascular Events in a Clinical Trial of Interleukin-1β Blockade., PMID:39862678
Post-Marketing Pharmacovigilance of Canakinumab from the FDA Adverse Event Reporting System (FAERS)., PMID:39861175
Background and Clinical Features of a Unique and Mysterious Autoinflammatory Disease, Schnitzler Syndrome., PMID:39859314