Catalog No.
KDB94403
Description
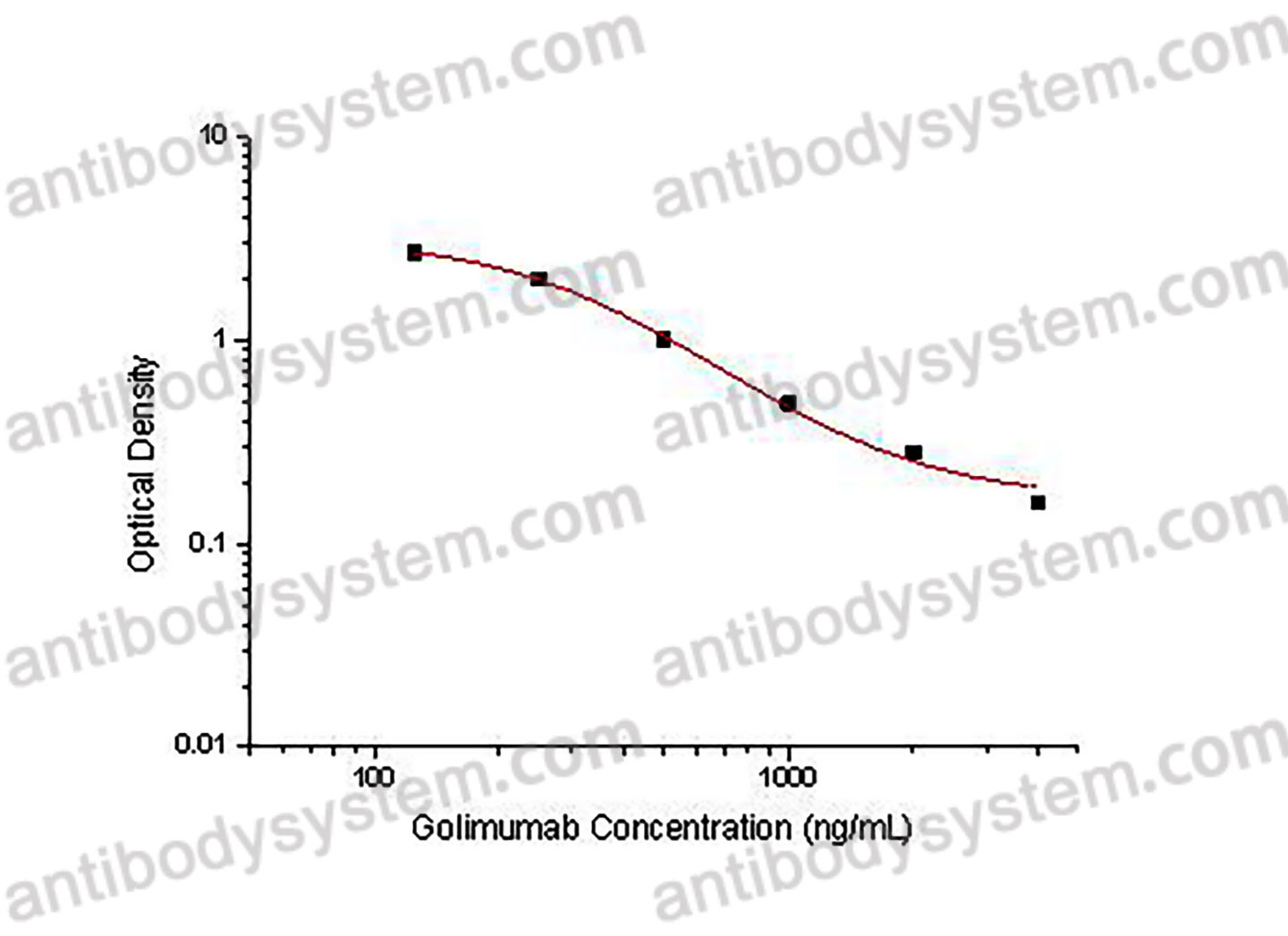
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human TNFa has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Golimumab in the sample competitively binds to the pre-coated protein with biotin-labeled Golimumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Golimumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Golimumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 4,000 ng/mL
Sensitivity
99.16 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1763.1
|
406.7
|
110.1
|
1796.4
|
392.4
|
88.7
|
|
Standard deviation
|
81.2
|
15.7
|
13.4
|
123.7
|
22.4
|
17.7
|
|
CV (%)
|
4.6
|
3.9
|
12.2
|
6.9
|
5.7
|
20.0
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CNTO 148, CAS: 476181-74-5
Background
Golimumab (Simponi®) is a human immunoglobulin G1? monoclonal antibody which is specific for pro-inflammatory cytokine, tumor necrosis factor-a (TNFa). In 2009, it was approved by FDA for the treatment of rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis in adult patients. Elevated levels of TNF are found in the synovial fluid of rheumatoid arthritis, including juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis patients and play an important role in both the pathologic inflammation and the joint destruction that are hallmarks of these diseases. Increased levels of TNF are also found in psoriasis (Ps) plaques. Golimumab binds to both the soluble and transmembrane bioactive forms of human TNF and prevent TNF from binding to its receptors and finally inhibits biological activity of TNF.
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Pharmacogenomics of TNF inhibitors., PMID:40469293
Experience of High Tibial Osteotomy for Patients with Rheumatoid Arthritis Treated with Recent Medication: A Case Series., PMID:40429328
The Relationship Between TNF-α Inhibitor Potency and HBV Reactivation in Patients With Rheumatic Disorders., PMID:40418092
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Golimumab retention in patients with psoriatic arthritis and axial spondyloarthritis: evidence from up to a decade of follow-up., PMID:40380936
Drug-associated infections and infestations in older adults with tumor necrosis factor-alpha inhibitors: a real-world retrospective and pharmacovigilance study., PMID:40371340
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559
Unveiling the differences: infection disorders associated with tumor necrosis factor α inhibitors in pediatric patients-a pharmacovigilance study (2004-2023)., PMID:40317305
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Racial Disparities in Utilization of Medications and Disease Outcomes in Inflammatory Bowel Disease Patients., PMID:40260307
Recommendations for the use of DMARDs in pregnancy and reproductive health for patients with rheumatic disease: A scoping review., PMID:40256995
Characterising infusion/injection-related reactions in patients with rheumatoid arthritis treated with biologic agents., PMID:40249052
Relapsing-remitting multiple sclerosis as a potential consequence of thalidomide treatment: A case report., PMID:40245782
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
Dual biological treatments in immune-mediated disorders: a single center experience., PMID:40200173
Rapid, Single-Step Monitoring of Monoclonal Antibody Bioavailability by Using a TNF-α-Based Multiepitope DNA Nanoswitch., PMID:40198205
Biosimilars versus biological therapy in inflammatory bowel disease: challenges and targeting strategies using drug delivery systems., PMID:40186719
Infection toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real-world study based on the US food and drug administration adverse events reporting system (FAERS)., PMID:40156444
Treatment of uveitis in Blau syndrome: A systematic review and meta-analysis., PMID:40147219
Abatacept, Golimumab, and Sarilumab as Selected Bio-Originator Disease-Modifying Antirheumatic Drugs with Diverse Mechanisms of Action in Their Current Use in Treatment., PMID:40142915
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Guselkumab versus golimumab in patients with active psoriatic arthritis and inadequate response to an initial tumor necrosis factor inhibitor: study protocol for EVOLUTION, a pragmatic, phase 3b, open-label, randomized, controlled effectiveness trial., PMID:40102973
Effect of TNF-α blockers on reducing the risk of dementia in rheumatoid arthritis: a nationwide cohort study., PMID:40095620
Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis., PMID:40091354
Golimumab-induced lichen planus pigmentosus in a patient with ulcerative colitis., PMID:40087062
Risk of hematologic malignancies in psoriasis and rheumatoid arthritis patients using long term TNF-α inhibitors: a retrospective nationwide study., PMID:40055388
Dose Escalation Patterns and Associated Costs of Advanced Therapies for Ulcerative Colitis in France and the United Kingdom: A Retrospective Database Analysis., PMID:40046402
Conducting a real-world study of Tumor Necrosis factor-alpha inhibitors-induced Systemic Lupus Erythematosus based on the FAERS database., PMID:40000785
In vitro Stability Study of a Panel of Commercial Antibodies at Physiological pH and Temperature as a Guide to Screen Biologic Candidate Molecules for the Potential Risk of In vivo Asparagine Deamidation and Activity Loss., PMID:39979532
Comparative Effectiveness and Safety of the JAK Inhibitors and Biologic Disease-Modifying Antirheumatic Drugs in Treating Children With Nonsystemic Juvenile Idiopathic Arthritis: A Bayesian Meta-Analysis of Randomized Controlled Trials., PMID:39964338
Cost-Effectiveness Analysis of Upadacitinib in Patients With Moderately to Severely Active Ulcerative Colitis in Greece., PMID:39954537
An update on the safety of biologic therapies for the treatment of polyarticular juvenile idiopathic arthritis., PMID:39946290
Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis., PMID:39941759
A Longitudinal Post-authorization Safety Study of Golimumab in Treatment of Ulcerative Colitis: A Cohort Study in Denmark and Sweden, 2013-2021., PMID:39913070
Incidence rates of tuberculosis and inflammatory bowel disease in patients with ankylosing spondylitis treated with biologics in Korea., PMID:39854270
Successful Treatment of Refractory Ulcerative Colitis With 5-Aminosalicylic Acid Intolerance and Biologic Therapy Resistance Using Combined Granulocyte and Monocyte Adsorptive Apheresis., PMID:39834662
Paradoxical Inflammatory Bowel Disease Induced by Golimumab in a Patient With Ankylosing Spondylitis: A Case Report and Systematic Review., PMID:39807347
Construct validity and responsiveness of ASAS Health Index assessed in two longitudinal studies of tumour necrosis factor alpha inhibitor initiation and dose reduction in patients with axial spondyloarthritis., PMID:39794273
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis., PMID:39757285
Treatment Persistence Among Anti-Tumor Necrosis Factor-experienced Patients With Ulcerative Colitis Switching to a Biologic With a Different Mode of Action or Cycling to Another Anti-Tumor Necrosis Factor Agent., PMID:39743427
Uncovering novel therapeutic clues for hypercoagulable active ulcerative colitis: novel findings from old data., PMID:39735422
Effectiveness and Treatment Persistence of Vedolizumab Compared to Anti-Tumour Necrosis Factor-α in Patients With Crohn's Disease: A Systematic Literature Review and Meta-Analysis., PMID:39707930
Persistence of advanced therapies in patients with inflammatory bowel disease: retrospective cohort study using a large healthcare claims database in Japan., PMID:39701922
Pulmonary toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real world study based on US food and drug administration adverse events reporting system (FAERS)., PMID:39695351
Interactions of warfarin with COVID-19 vaccine/drugs, monoclonal antibodies, and targeted anticancer agents from real-world data mining., PMID:39679378
Association Between Biologics and Janus Kinase Inhibitors With Inflammatory Bowel Disease as Paradoxical Reactions: A Real-World Assessment., PMID:39676727
Efficacy and Safety of Tumor Necrosis Factor Inhibitors, Interleukin-17 Inhibitors, and Janus Kinase Inhibitors in Patients with Non-Radiographic Axial Spondyloarthritis: A Systematic Review and Network Meta-Analysis., PMID:39657601