Catalog No.
KDB94402
Description
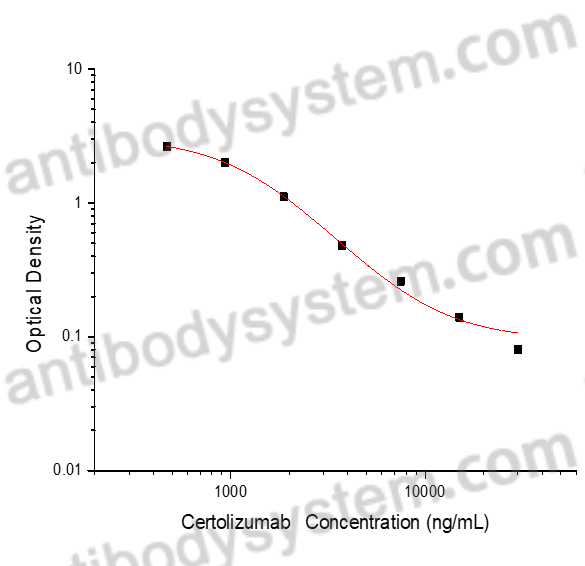
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human TNFa has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Certolizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Certolizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Certolizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Certolizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
468.75 - 30,000 ng/mL
Sensitivity
329.66 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
19649.7
|
4203.7
|
1503.8
|
16500.1
|
3681.2
|
1240.7
|
|
Standard deviation
|
2286.2
|
360.4
|
128.4
|
2008.9
|
422.3
|
95.4
|
|
CV (%)
|
11.6
|
8.6
|
8.5
|
12.2
|
11.5
|
7.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
CDP870, PHA-738144, Certolizumab pegol, CAS: 428863-50-7
Background
Certolizumab pegol is a recombinant, humanized F(ab)’ fragment of an antibody conjugated to polyethylene glycol to enhance its plasma half-life. It does not contain an FC region and therefore does not cause antibody-dependent cell-mediated or complement-dependent cytotoxicity as described for infliximab, etanercept, and adalimumab. Another difference is that certolizumab pegol preferentially penetrates inflamed tissue compared to noninflamed tissue, and does so to a greater extent than adalimumab or infliximab. It is directed against soluble and membrane-associated human TNF-α and was approved for the treatment of Crohn’s disease that does not respond to conventional therapy in 2008 and for RA in 2009.
Certolizumab pegol to prevent adverse pregnancy outcomes in patients with antiphospholipid syndrome and lupus anticoagulant (IMPACT): results of a prospective, single-arm, open-label, phase 2 trial., PMID:40483169
Glucocorticoid treatment in early rheumatoid arthritis is independently associated with increased PCSK9 levels: data from a randomised controlled trial., PMID:40480650
Gender perspective in the management of psoriasis., PMID:40470625
Pharmacogenomics of TNF inhibitors., PMID:40469293
Predicting Clinical Response to Monoclonal TNF Inhibitors in Rheumatoid Arthritis: A Transcriptomic Approach Based on Transmembrane TNF Reverse Signaling and Nrf2 Activation., PMID:40428231
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Influence of Rheumatoid Factors on the Efficacy of TNF Inhibitor Therapy in Patients with Rheumatoid Arthritis., PMID:40377857
Cardiovascular Outcomes of Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis: A Review of the Current Evidence., PMID:40376321
Drug-associated infections and infestations in older adults with tumor necrosis factor-alpha inhibitors: a real-world retrospective and pharmacovigilance study., PMID:40371340
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Aggregate Distributional Cost-Effectiveness Analysis of Biologics for the Treatment of Ankylosing Spondylitis in Chile., PMID:40329067
Novel Small-Molecule Treatment and Emerging Biological Therapy for Psoriasis., PMID:40299379
Racial Disparities in Utilization of Medications and Disease Outcomes in Inflammatory Bowel Disease Patients., PMID:40260307
Transitional and CD21- PD-1+ B cells are associated with remission in early rheumatoid arthritis., PMID:40259340
Recommendations for the use of DMARDs in pregnancy and reproductive health for patients with rheumatic disease: A scoping review., PMID:40256995
Triple biologic therapy for refractory Crohn's disease., PMID:40251965
A Case of Rectus Sheath Hematoma Caused by Self-Injection of Certolizumab Pegol., PMID:40196069
Discontinuation vs. continuation of concomitant methotrexate in patients with rheumatoid arthritis on certolizumab pegol: results from a randomised, controlled trial., PMID:40188084
Infection toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real-world study based on the US food and drug administration adverse events reporting system (FAERS)., PMID:40156444
Certolizumab-Induced Urticarial Vasculitis: A Case Report., PMID:40143392
Biological Disease-Modifying Antirheumatic Drugs Decrease Uric Acid Levels in the Sera of Patients with Psoriatic Arthritis., PMID:40136396
Pharmacovigilance study and genetic target prediction analysis of FDA adverse event reports (FAERS) for drug-induced sinusitis., PMID:40128146
Real-Life Analysis of Therapeutic Management and Its Correlation with the Dermatology Life Quality Index Score in 108 Patients with Pustular Psoriasis: An Italian Monocenter Study., PMID:40117644
Cost-effectiveness analysis of subcutaneous biosimilar tocilizumab in patients with rheumatoid arthritis in Spain., PMID:40113553
Rheumatoid Factor Predicts Long-Term Retention Associated With Effectiveness of Certolizumab Pegol in Patients With Rheumatoid Arthritis: A Two-Center Retrospective Study., PMID:40099319
Anti-Rheumatic potential of biological DMARDS and protagonistic role of bio-markers in early detection and management of rheumatoid arthritis., PMID:40091354
Certolizumab enhances spinal cord injury recovery in rats through inhibition of the TNF-α signaling pathway and neuronal apoptosis., PMID:40009347
Conducting a real-world study of Tumor Necrosis factor-alpha inhibitors-induced Systemic Lupus Erythematosus based on the FAERS database., PMID:40000785
Rheumatoid factors revisited in the age of biologic therapy., PMID:39982406
Risankizumab and Certolizumab Pegol Dual-Targeted Therapy for Crohn's Disease and Axial Spondyloarthritis: A Case Report., PMID:39981170
The use of aptamers as therapeutic inhibitors and biosensors of TNF-alpha., PMID:39971069
Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis., PMID:39941759
Systematic review and bayesian network meta-analysis: comparative efficacy and safety of six commonly used biologic therapies for moderate-to-severe Crohn's disease., PMID:39911832
Certolizumab Pegol Treatment in Patients With Crohn's Disease: Final Safety Data From the SECURE Registry., PMID:39895830
Hidradenitis Suppurativa Treatment During Pregnancy and Lactation: Navigating Challenges., PMID:39887706
Management of Anal Fistula with Crohn's Disease., PMID:39882221
Exploring the neuroprotective potential of immunosuppressants in Parkinson's disease., PMID:39874798
Pharmacovigilance Pregnancy Data in a Population of Japanese Patients With Chronic Inflammatory Disease Exposed to Certolizumab Pegol., PMID:39812078
Certolizumab pegol in severe hidradenitis suppurativa in pregnancy: A case report., PMID:39803378
What is rheumatoid factor? From screening to personalized management., PMID:39792161
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis., PMID:39757285
Effects of Ab501 (certolizumab mice equivalent) in arthritis induced bone loss., PMID:39754728
Impaired coagulation parameters in early RA are restored by effective antirheumatic therapy: a prospective pilot study., PMID:39740931
Effectiveness and Treatment Persistence of Vedolizumab Compared to Anti-Tumour Necrosis Factor-α in Patients With Crohn's Disease: A Systematic Literature Review and Meta-Analysis., PMID:39707930
Certolizumab pegol for plaque psoriasis in women of childbearing potential, pregnant or breastfeeding in clinical settings: One-year outcomes from the international noninterventional CIMREAL study., PMID:39699934
Pulmonary toxicity assessment of tumor necrosis factor α inhibitors in the treatment of IBD: a real world study based on US food and drug administration adverse events reporting system (FAERS)., PMID:39695351
Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet's Disease., PMID:39685848
Association Between Biologics and Janus Kinase Inhibitors With Inflammatory Bowel Disease as Paradoxical Reactions: A Real-World Assessment., PMID:39676727