Catalog No.
KDB94401
Description
PRINCIPLE OF THE ASSAY
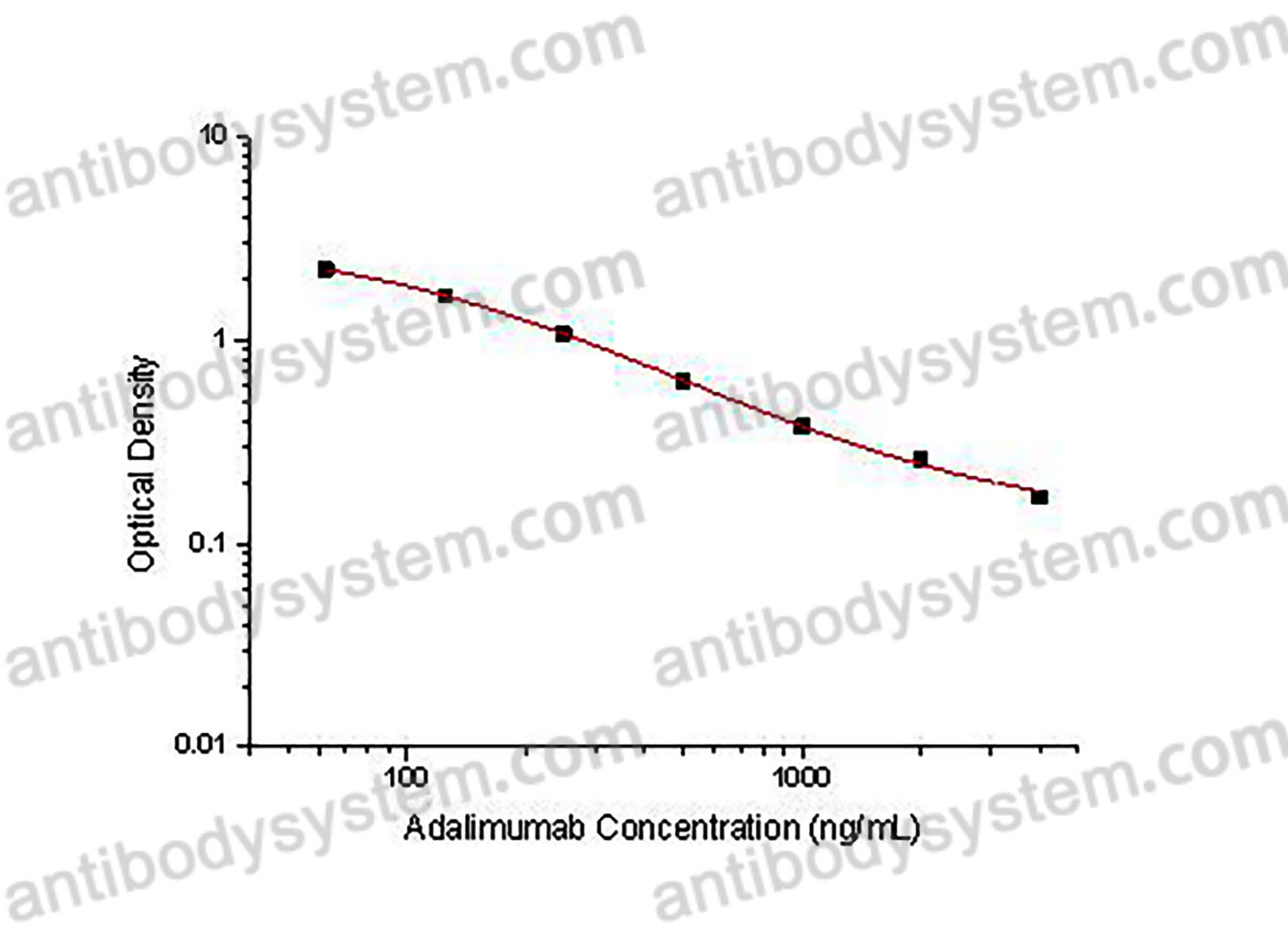
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human TNFa has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Adalimumab in the sample competitively binds to the pre-coated protein with biotin-labeled Adalimumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Adalimumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Adalimumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
102.26 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1568.7
|
416.8
|
229.0
|
1671.5
|
349.9
|
216.2
|
|
Standard deviation
|
129.6
|
50.7
|
21.5
|
136.9
|
40.7
|
22.4
|
|
CV (%)
|
8.3
|
12.2
|
9.4
|
8.2
|
11.6
|
10.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ABP 501, D2E7, Adalimumab, CAS: 331731-18-1
Background
Adalimumab is a recombinant human IgG1 monoclonal antibody specific for Tumor Necrosis Factor-Alpha (TNF-a) and is used to treat rheumatic arthritis, intestinal disorders, dermatological diseases and cancer. Adalimumab specifically binds to TNF alpha and blocks its interaction with p55 and p75 cell surface TNF receptors and reduces the inflammation and subsequently improves the patient’s health. Drug level quantification can be important to adapt patient prescription or to switch to an alternative TNF inhibitor drug.
Therapeutic Potential of Upadacitinib in Adolescents with Idiopathic Chronic Panuveitis Who Lost Response to Adalimumab., PMID:40515519
Association between rapid and sustained remission and clinician- and patient-reported outcomes in patients with rheumatoid arthritis: post hoc analysis of data from the SELECT-COMPARE study., PMID:40514717
Retinal venous occlusion in drug-induced lupus., PMID:40514329
Long-Term Effectiveness and Safety of Weekly Adalimumab in Refractory Non-Infectious Uveitis., PMID:40511491
A physiologically based pharmacokinetic model to describe [89Zr]Zr-DFO-Adalimumab specific activity after intravitreal administration to rats with endotoxin-induced uveitis., PMID:40505897
Development and validation of a novel Endoscopic ulcer Activity Score for Evaluation of Crohn's Disease (EASE-CD) using data from two randomised controlled trials., PMID:40505666
Summary of Research: Immunogenicity of Adalimumab Reference Product and Adalimumab-adbm in Patients with Rheumatoid Arthritis, Crohn's Disease, and Chronic Plaque Psoriasis: A Pooled Analysis of the VOLTAIRE trials., PMID:40498294
Upadacitinib for ulcerative colitis and pyoderma gangrenosum in a patient with schizophrenia on long-term risperidone: A case report., PMID:40495949
Efficacy and Safety of ABBV-154 for the Treatment of Active Rheumatoid Arthritis: A Phase 2b, Randomized, Placebo-Controlled Trial., PMID:40495262
Deep learning-based ranking method for subgroup and predictive biomarker identification in patients., PMID:40494908
A single-cell RNA-seq dataset of synovial fluid from rheumatoid arthritis treated with TNF-α/JAK inhibitor., PMID:40494845
Comparison and analysis of biological drug consumption in two Italian hospital settings: Governance actions and prescribing appropriateness., PMID:40494065
Multiple Switches Between Adalimumab-fkjp and Reference Adalimumab in Moderate-to-Severe Chronic Plaque Psoriasis: A Multicenter, Double-Blind, Parallel Group, Randomized Clinical Trial for Interchangeability., PMID:40493334
Management of Pediatric Acne Vulgaris and Hidradenitis Suppurativa in Minoritized and Underserved Populations., PMID:40489363
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Treatment of refractory pityriasis rubra pilaris with biologic therapy: a case series., PMID:40488550
Reply to Correspondence to Recurrence Risk in Pediatric Non-Infectious Uveitis during Adalimumab Tapering., PMID:40485634
Drug survival, long-term safety and efficacy of anti-TNF-alpha biosimilars: a monocentric retrospective study., PMID:40485573
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Tumor necrosis factor α inhibitor-induced alopecia in pediatric patients: a cohort of 20 patients and review of the literature., PMID:40481366
A Survey of Diagnostic and Management Practices in Retinal Vasculitis: International Uveitis Study Group (IUSG) Retinal Vasculitis Study (ReViSe)-Report 5., PMID:40478525
Effects of Adalimumab on Mitochondria of Psoriatic Mesenchymal Stem Cells., PMID:40478194
Case Report: Kyrieleis plaques in an unusual Behcet's disease uveitis., PMID:40475959
Case Report: Effectiveness of deucravacitinib in chronic recurrent multifocal osteomyelitis and concomitant psoriasis., PMID:40475785
Pharmacotherapy for non-infectious uveitis: spotlight on phase III clinical trials of locally injected or implanted therapeutics and systemic immunomodulatory drugs., PMID:40473986
Anti-tumor Necrosis Factor-Alpha (TNF-α)-Induced Hypertrophic Lichen Planus: A Case Report., PMID:40470457
Pharmacogenomics of TNF inhibitors., PMID:40469293
Lupus panniculitis secondary to treatment with adalimumab in a patient with rheumatoid arthritis., PMID:40466251
Adalimumab Monotherapy vs Adalimumab With Methotrexate for Psoriasis., PMID:40465280
High-Throughput Human Gut Immune Co-Culture Model for Evaluating Inflammatory Bowel Disease Anti-Inflammatory Therapies., PMID:40462955
Benefit-risk analysis of upadacitinib versus adalimumab in patients with rheumatoid arthritis and higher or lower risk of cardiovascular disease., PMID:40461266
Severe paradoxical generalized pustular psoriasis induced by adalimumab biosimilar successfully treated with brodalumab., PMID:40457932
Correspondence to Recurrence Risk in Pediatric Non-Infectious Uveitis during Adalimumab Tapering: An International Multicenter Retrospective Study., PMID:40456698
Treatment of Autoimmune Enteropathy With Vedolizumab., PMID:40452653
Immunoglobulin A vasculitis and pustular psoriasis precipitated by Tawon Liar: a case report., PMID:40448236
Letter to the Editor: Assessing early therapeutic drug monitoring of adalimumab as a predictor of treatment efficacy and immunogenicity in rheumatic diseases: "early therapeutic drug monitoring of adalimumab"., PMID:40445523
Clinical Response to Adalimumab Therapy and Its Determinants in Patients With Radiographic Axial Spondyloarthritis: A Prospective Real-World Study in Taiwan., PMID:40443026
Cross-phenotype genome-wide association study supports shared genetic etiology between skin and gastrointestinal tract diseases., PMID:40441863
Physician Burden and Time Delays in Initiating Immunomodulatory Therapy for Non-infectious Uveitis and Inflammatory Eye Diseases., PMID:40441503
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Uveitis in Adults: A Review., PMID:40434762
Failure of primary immunosuppressive agents in uveitis., PMID:40434459
JAK-STAT inhibitors in noninfectious uveitis - A review., PMID:40434456
Optimizing Biologic Therapy for the Prevention of Post-Operative Recurrence in Crohn's Disease: Current Evidence and Future Perspectives., PMID:40427059
Subconjunctival Adalimumab for Noninfectious Uveitis: A Prospective Pilot Study., PMID:40423087
Anti-Adalimumab Antibodies Purified from Juvenile Idiopathic Arthritis Patients: Kinetic Characterization Among Biosimilars., PMID:40422017
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Comparative efficacy, safety and immunogenicity of biosimilars and their reference biologic drugs in ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials., PMID:40418343