Catalog No.
KDB91701
Description
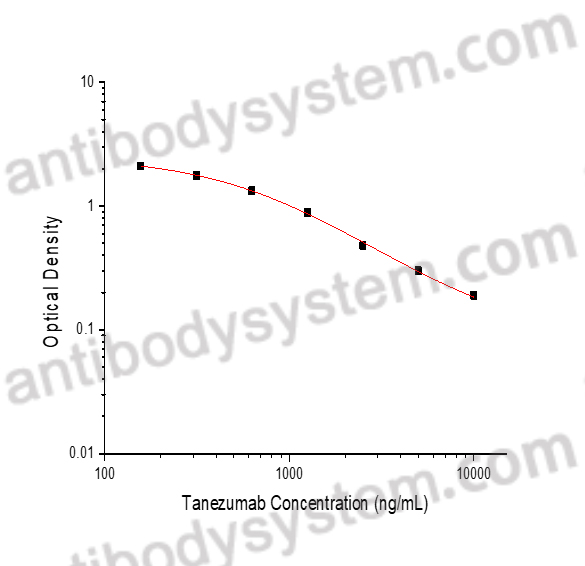
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human NGF has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tanezumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tanezumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tanezumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Tanezumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
128.01 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
4861.0
|
1011.8
|
253.3
|
5343.4
|
1264.1
|
293.1
|
|
Standard deviation
|
218.8
|
64.1
|
29.0
|
523.4
|
167.7
|
51.5
|
|
CV (%)
|
4.5
|
6.3
|
11.4
|
9.8
|
13.3
|
17.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
PF-04383119, RN624, CAS: 880266-57-9
Background
Tanezumab (RN624) is a recombinant humanized anti- nerve growth factor (NGF) monoclonal antibody (IgG2 type) that works by inhibiting the binding of NGF to its receptors. It was developed by Pfizer as a treatment for pain.
Associations between testosterone and knee and hand osteoarthritis among males and females from the general population., PMID:40221126
Metabolic syndrome, radiographic osteoarthritis progression and chronic pain of the knee among men and women from the general population: The Rotterdam study., PMID:39288696
Re: Re: Laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs., PMID:38885831
The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain., PMID:38752946
Effect size varies based on calculation method and may affect interpretation of treatment effect: an illustration using randomised clinical trials in osteoarthritis., PMID:38650049
Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients., PMID:37954824
Characterization of adverse joint outcomes in patients with osteoarthritis treated with subcutaneous tanezumab., PMID:37652258
Prediction of relative change in free nerve growth factor following subcutaneous administration of tanezumab, a novel monoclonal antibody to nerve growth factor., PMID:37470295
Peripheral Nerve Safety of Nerve Growth Factor Inhibition by Tanezumab: Pooled Analyses of Phase III Clinical Studies in Over 5000 Patients with Osteoarthritis., PMID:37460782
A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects With Cancer Pain Due to Bone Metastasis., PMID:37343145
Role of imaging for eligibility and safety of a-NGF clinical trials., PMID:37284331
Examining the Relationships Among Treatment, Pain, and Physical Function in Patients With Osteoarthritis: A Mediation-Modeling Approach., PMID:36806283
The Current Role of Disease-modifying Osteoarthritis Drugs., PMID:36793668
Predicting Treatment Responses in Patients With Osteoarthritis: Results From Two Phase III Tanezumab Randomized Clinical Trials., PMID:36621827
[Clinical characteristics of immune checkpoint inhibitor-related type 1 diabetes mellitus]., PMID:36594136
Different Dosage Regimens of Tanezumab for the Treatment of Chronic Low Back Pain: A Meta-analysis of Randomized Controlled Trials., PMID:36542785
Time to first and sustained improvement in WOMAC domains among patients with osteoarthritis receiving tanezumab., PMID:36474952
A Two-Step, Trajectory-Focused, Analytics Approach to Attempt Prediction of Analgesic Response in Patients with Moderate-to-Severe Osteoarthritis., PMID:36301512
Nerve Growth Factor (NGF) Encourages the Neuroinvasive Potential of Pancreatic Cancer Cells by Activating the Warburg Effect and Promoting Tumor Derived Exosomal miRNA-21 Expression., PMID:36285300
Inter-Reader Consistency and Exclusionary Findings During Radiographic Screening for Phase 3 Trials of Tanezumab in Patients With Osteoarthritis., PMID:38343426
Design of a randomized, placebo-controlled, phase 2 study evaluating the safety and efficacy of tanezumab for treatment of schwannomatosis-related pain., PMID:36038003
Efficacy and safety of tanezumab, NSAIDs, and placebo in patients with moderate to severe hip or knee osteoarthritis and a history of depression, anxiety, or insomnia: post-hoc analysis of phase 3 trials., PMID:35980115
Clinical Meaningfulness of Response to Tanezumab in Patients with Chronic Low Back Pain: Analysis From a 56-Week, Randomized, Placebo- and Tramadol-Controlled, Phase 3 Trial., PMID:35962939
Characterizing 16-Week Responder Profiles Using Group-Based Trajectory Modeling in Over 4300 Clinical Trial Participants Receiving Pharmaceutical Treatment for Moderate to Severe Osteoarthritis., PMID:35960482
Trends of Dispensed Opioids in Catalonia, Spain, 2007-19: A Population-Based Cohort Study of Over 5 Million Individuals., PMID:35754470
Monoclonal Antibody Therapy for the Treatment of Interstitial Cystitis., PMID:35619987
Efficacy, General Safety, and Joint Safety of Tanezumab in Japanese Patients with Osteoarthritis: Subgroup Analyses from Two Randomized, Phase 3 Studies., PMID:35538185
Efficacy and Safety of Anti-Nerve Growth Factor Antibody Therapy for Hip and Knee Osteoarthritis: A Meta-analysis., PMID:35494494
Gout and Hospital Admission for Ambulatory Care-Sensitive Conditions: Risks and Trajectories., PMID:35428711
Analgesic effects of nerve growth factor-directed monoclonal antibody on diabetic neuralgia in an animal model., PMID:35417079
Tanezumab for the treatment of osteoarthritis pain., PMID:35412532
Comorbidities in osteoarthritis (ComOA): a combined cross-sectional, case-control and cohort study using large electronic health records in four European countries., PMID:35387809
Observed efficacy and clinically important improvements in participants with osteoarthritis treated with subcutaneous tanezumab: results from a 56-week randomized NSAID-controlled study., PMID:35351194
Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets., PMID:35295464
[Osteoarthritis: what's new?]., PMID:35291041
WOMAC Meaningful Within-patient Change: Results From 3 Studies of Tanezumab in Patients With Moderate-to-severe Osteoarthritis of the Hip or Knee., PMID:35232805
Neurological safety of subcutaneous tanezumab versus NSAID in patients with osteoarthritis., PMID:35217440
Development of radiographic classification criteria for hand osteoarthritis: a methodological report (Phase 2)., PMID:35121640
Population pharmacokinetics of tanezumab following intravenous or subcutaneous administration to patients with osteoarthritis or chronic low back pain., PMID:35112378
Impact of tanezumab on health status, non-work activities and work productivity in adults with moderate-to-severe osteoarthritis., PMID:35105318
Based on minimal clinically important difference values, a moderate dose of tanezumab may be a better option for treating hip or knee osteoarthritis: a meta-analysis of randomized controlled trials., PMID:35069811
Effectiveness of Various Dosages and Administration Methods of Tanezumab for the Treatment of Pain in Knee and Hip Osteoarthritis: a Network Meta-Analysis., PMID:34819241
Nerve Growth Factor (NGF) Inhibitors and Related Agents for Chronic Musculoskeletal Pain: A Comprehensive Review., PMID:34807432
Tanezumab for chronic low back pain: a long-term, randomized, celecoxib-controlled Japanese Phase III safety study., PMID:34786956
Different Drugs for the Treatment of Painful Diabetic Peripheral Neuropathy: A Meta-Analysis., PMID:34777192
Association of Tramadol vs Codeine Prescription Dispensation With Mortality and Other Adverse Clinical Outcomes., PMID:34665205
Preparation and characterization of a high-affinity monoclonal antibody against nerve growth factor., PMID:34627999
Gender, age, disease severity, body mass index and diabetes may not affect response to subcutaneous tanezumab in patients with osteoarthritis after 16 weeks of treatment. A subgroup analysis of placebo-controlled trials., PMID:34626502
Relative Efficacy and Safety of Tanezumab for Osteoarthritis: A Systematic Review and Meta-analysis of Randomized-Controlled Trials., PMID:34608021
Single and Composite Endpoints of Within-Patient Improvement in Symptoms: Pooled Tanezumab Data in Patients with Osteoarthritis., PMID:34606077