Catalog No.
KDB86901
Description
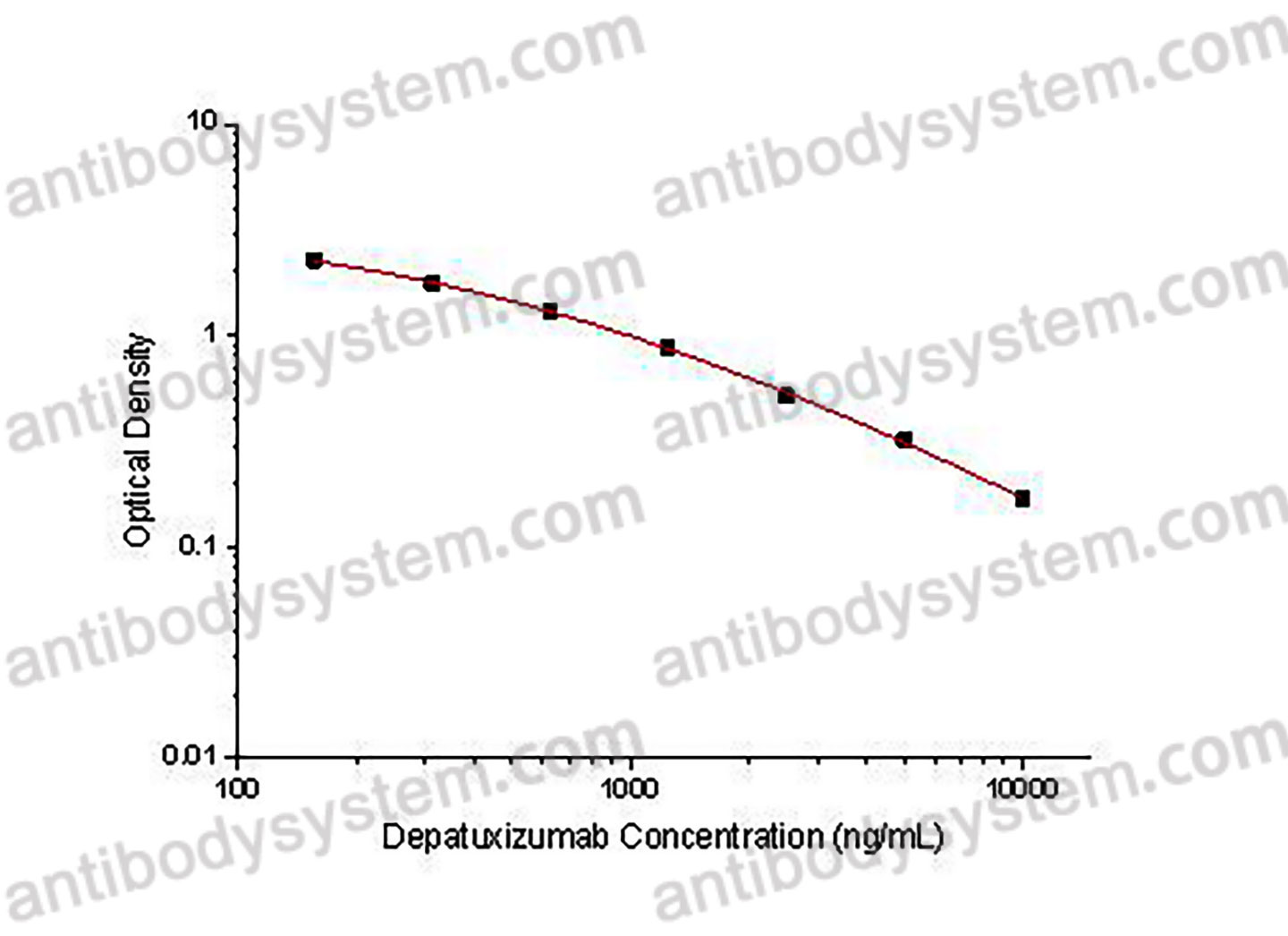
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human EGFR has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Depatuxizumab in the sample competitively binds to the pre-coated protein with biotin-labeled Depatuxizumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Depatuxizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Depatuxizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
79.88 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
5788.3
|
1295.9
|
285.7
|
6264.1
|
1396.3
|
236.2
|
|
Standard deviation
|
284.2
|
122.1
|
31.5
|
334.7
|
105.2
|
32.8
|
|
CV (%)
|
4.9
|
9.4
|
11.0
|
5.3
|
7.5
|
13.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
ABT-806, ABT-414, unconjugate: ABT-806, CAS: 1471999-69-5
Background
Depatuxizumab Mafodotin (Depatux-M; ABT-414) is an antibody-drug conjugate consisting of a specific antibody against activated EGFR and a cytotoxic agent with antimicrotubule activity. The INTELLANCE 2/EORTC 1410 phase 2 trial produced interesting results for the combination regimen of Depatux-M and temozolomide in EGFR-amplified glioblastoma patients at first recurrence. For the first time worldwide, our work investigated the clinical outcome and safety of this combination in a real-life population.
Bystander Effects, Pharmacokinetics, and Linker-Payload Stability of EGFR-Targeting Antibody-Drug Conjugates Losatuxizumab Vedotin and Depatux-M in Glioblastoma Models., PMID:38743766
Focus on current and emerging treatment options for glioma: A comprehensive review., PMID:38689623
Efficacy of depatuxizumab mafodotin (ABT-414) in preclinical models of head and neck cancer., PMID:38375733
A Phase 3b Study for Management of Ocular Side Effects in Patients with Epidermal Growth Factor Receptor-Amplified Glioblastoma Receiving Depatuxizumab Mafodotin., PMID:37257422
Understanding the activity of antibody-drug conjugates in primary and secondary brain tumours., PMID:37085569
Convection enhanced delivery of EGFR targeting antibody-drug conjugates Serclutamab talirine and Depatux-M in glioblastoma patient-derived xenografts., PMID:36071925
Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial., PMID:35849035
Characterization and Potential Mitigation of Corneal Effects in Nonclinical Toxicology Studies in Animals Administered Depatuxizumab Mafodotin., PMID:35537481
Safety and efficacy of depatuxizumab mafodotin in Japanese patients with malignant glioma: A nonrandomized, phase 1/2 trial., PMID:34609773
Ocular surface toxicity of depatuxizumab mafoditin (ABT-414): case reports., PMID:34586240
Tumor volumes as a predictor of response to the anti-EGFR antibody drug conjugate depatuxizumab mafadotin., PMID:34549181
Veliparib in Combination With Platinum-Based Chemotherapy for First-Line Treatment of Advanced Squamous Cell Lung Cancer: A Randomized, Multicenter Phase III Study., PMID:34436928
Depatuxizumab Mafodotin (Depatux-M) Plus Temozolomide in Recurrent Glioblastoma Patients: Real-World Experience from a Multicenter Study of Italian Association of Neuro-Oncology (AINO)., PMID:34204877
Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma., PMID:34050676
Impact of depatuxizumab mafodotin on health-related quality of life and neurological functioning in the phase II EORTC 1410/INTELLANCE 2 trial for EGFR-amplified recurrent glioblastoma., PMID:33601293
Synergistic therapeutic benefit by combining the antibody drug conjugate, depatux-m with temozolomide in pre-clinical models of glioblastoma with overexpression of EGFR., PMID:33517558
A Phase 1 and Biodistribution Study of ABT-806i, an 111In-Radiolabeled Conjugate of the Tumor-Specific Anti-EGFR Antibody ABT-806., PMID:33509972
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate., PMID:33201054
Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study., PMID:33154952
Targeting Multiple EGFR-expressing Tumors with a Highly Potent Tumor-selective Antibody-Drug Conjugate., PMID:32847977
Corneal side effects induced by EGFR-inhibitor antibody-drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study., PMID:32550861
Depatuxizumab Mafodotin (ABT-414)-induced Glioblastoma Cell Death Requires EGFR Overexpression, but not EGFRY1068 Phosphorylation., PMID:32371586
A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR., PMID:32189093
A troublesome burden, the amplification of EGFR in glioblastoma!, PMID:32144420
INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma., PMID:31747009
How we treat glioblastoma., PMID:31297242
An Integrated Population Pharmacokinetic Model Versus Individual Models of Depatuxizumab Mafodotin, an Anti-EGFR Antibody Drug Conjugate, in Patients With Solid Tumors Likely to Overexpress EGFR., PMID:30990907
Antibodies to watch in 2019., PMID:30516432
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: Retraction., PMID:30102618
Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial., PMID:29982805
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: [RETRACTED]., PMID:29794826
Efficacy and safety results of depatuxizumab mafodotin (ABT-414) in patients with advanced solid tumors likely to overexpress epidermal growth factor receptor., PMID:29533458
Characterization of ABBV-221, a Tumor-Selective EGFR-Targeting Antibody Drug Conjugate., PMID:29483208
Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma., PMID:29449271
Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments., PMID:29116596
Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma., PMID:29077941
Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study., PMID:29075855
Population Pharmacokinetics of ABT-806, an Investigational Anti-Epidermal Growth Factor Receptor (EGFR) Monoclonal Antibody, in Advanced Solid Tumor Types Likely to Either Over-Express Wild-Type EGFR or Express Variant III Mutant EGFR., PMID:25761639
Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody., PMID:25731184